Research on the optimization of the composition and technology of combined tablets of bisoprolol fumarate with indapamide
DOI:
https://doi.org/10.15587/2519-4852.2025.347945Keywords:
tablets, optimization, technology, excipients, direct compression, pharmaco-technological indicators, regression model, Quality by Design, response surface methodologyAbstract
The aim. The study was focused on the development and optimization of a rational tablet formulation containing bisoprolol fumarate and indapamide by applying a response surface methodology to ensure the required pharmaco-technological and biopharmaceutical characteristics of the final dosage form.
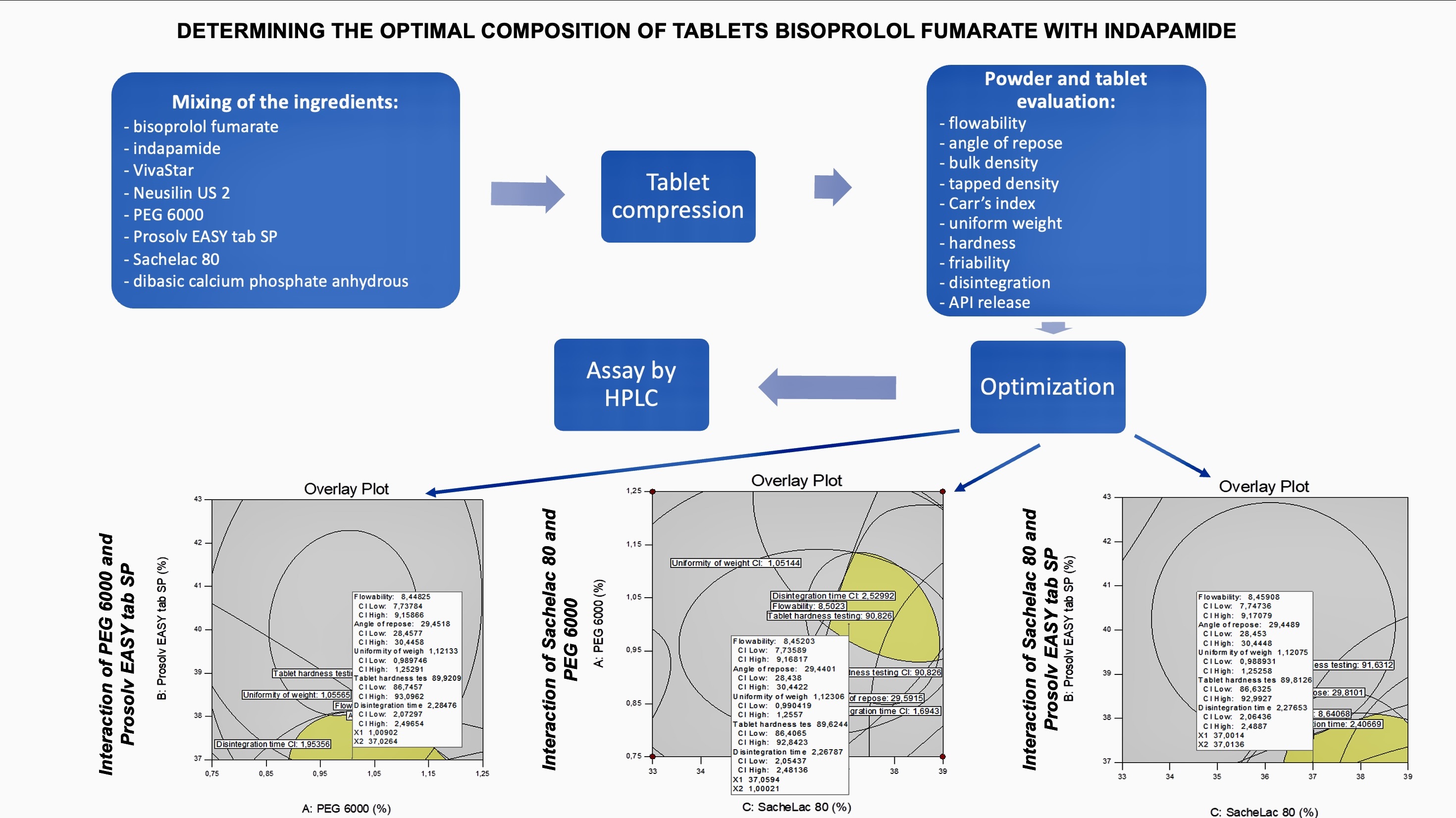
Materials and methods. The central composite design was used to establish the relation between independent variables, such as, quantity of PEG 6000, quantity of Prosolv EASYtab SP, quantity of Sachelac 80 and dependent variables, such as flowability, bulk density, tapped density, angle of repose, Carr’s index, uniformity of weight, friability, tablet hardness and disintegration time in order to obtain the optimal formulation using response surface methodology. Tablets were prepared by direct compression method. Quantitative determination of APIs in tablets was quantified by HPLC with UV detection at 220 nm.
Results. After generating the polynomial equations that relate the dependent and independent variables, the process was optimized for five responses. It was found that the tablet contained 1% PEG 6000, 37% Prosolv EASYtab SP, and 37% Sachelac 80 was a better formulation in terms of hardness (89 N), uniformity of weight (1.1%), friability (0.20%) and rapid disintegration (2.3 min). The experimental values of the dissolution of optimized tablets showed 95.6% release of bisoprolol fumarate and 99.7% release of indapamide. The quantitative content of active ingredients (bisoprolol fumarate and indapamide) in the developed tablets meets the requirements of the State Pharmacopoeia of Ukraine.
Conclusions. The study enabled the development of an optimized formulation and manufacturing process for combined bisoprolol fumarate and indapamide tablets, ensuring compliance with pharmaco-technological standards and demonstrating the applicability of response surface methodology for formulation design
Supporting Agency
- The work was carried out within the framework of the initiative search of research topics of the I. Horbachevsky Ternopil National Medical University in the absence of involvement of additional funding sources.
References
- Cardiovascular diseases (CVDs) (2025). World Health Organization. Available at: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)
- Pallarés-Carratalá, V., Ruiz-García, A., Serrano-Cumplido, A., Arranz-Martínez, E., Divisón-Garrote, J. A., Moyá-Amengual, A. et al. (2023). Prevalence Rates of Arterial Hypertension According to the Threshold Criteria of 140/90 or 130/80 mmHg and Associated Cardiometabolic and Renal Factors: SIMETAP-HTN Study. Medicina, 59 (10), 1846. https://doi.org/10.3390/medicina59101846
- Mancia, G., Kreutz, R., Brunström, M., Burnier, M., Grassi, G., Januszewicz, A. et al. (2023). 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension. Journal of Hypertension, 41 (12), 1874–2071. https://doi.org/10.1097/hjh.0000000000003480
- World Health Organization. STEPS: Prevalence of noncommunicable disease risk factors in Ukraine 2019. (2020). World Health Organization. Available at: https://apps.who.int/iris/bitstream/handle/10665/336642/WHO-EURO-2020-1468-41218-56060-eng.pdf?sequence=1&isAllowed=y
- Zhou, B., Carrillo-Larco, R. M., Danaei, G., Riley, L. M., Paciorek, C. J., Stevens, G. A. et al. (2021). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. The Lancet, 398 (10304), 957–980. https://doi.org/10.1016/s0140-6736(21)01330-1
- Zhou, B., Perel, P., Mensah, G. A., Ezzati, M. (2021). Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nature Reviews Cardiology, 18 (11), 785–802. https://doi.org/10.1038/s41569-021-00559-8
- Taddei, S., Tsabedze, N., Tan, R.-S. (2024). β-blockers are not all the same: pharmacologic similarities and differences, potential combinations and clinical implications. Current Medical Research and Opinion, 40 (1), 15–23. https://doi.org/10.1080/03007995.2024.2318058
- Sabidó, M., Hohenberger, T., Grassi, G. (2018). Pharmacological intervention in hypertension using beta-blockers: Real‐world evidence for long-term effectiveness. Pharmacological Research, 130, 191–197. https://doi.org/10.1016/j.phrs.2018.01.010
- Marti, H.-P., Pavía López, A. A., Schwartzmann, P. (2024). Safety and tolerability of β-blockers: importance of cardioselectivity. Current Medical Research and Opinion, 40 (1), 55–62. https://doi.org/10.1080/03007995.2024.2317433
- Mahfoud, F., Wang, J., Ray, S. (2024). The current position of β-blockers in hypertension: guidelines and clinical practice. Current Medical Research and Opinion, 40 (1), 25–32. https://doi.org/10.1080/03007995.2024.2318003
- Morales-Olivas, F. J. (2024). Diuretics use in the management of hypertension. Hipertensión y Riesgo Vascular, 41 (3), 186–193. https://doi.org/10.1016/j.hipert.2024.03.004
- Ernst, M. E., Fravel, M. A. (2022). Thiazide and the Thiazide-Like Diuretics: Review of Hydrochlorothiazide, Chlorthalidone, and Indapamide. American Journal of Hypertension, 35 (7), 573–586. https://doi.org/10.1093/ajh/hpac048
- Song, W., Cun, D., Quan, P., Liu, N., Chen, Y., Cui, H. et al. (2015). Dual-directional regulation of drug permeating amount by combining the technique of ion-pair complexation with chemical enhancers for the synchronous permeation of indapamide and bisoprolol in their compound patch through rabbit skin. European Journal of Pharmaceutics and Biopharmaceutics, 91, 59–65. https://doi.org/10.1016/j.ejpb.2015.01.025
- Ansari, M. T., Alahmed, T. A. A., & Sami, F. N.; Jain, K., Bajwa, N. (Eds.) (2024). Quality by Design (QbD) Concept for Formulation of Oral Formulations for Tablets. Introduction to Quality by Design (QbD). Springer, 161–184. https://doi.org/10.1007/978-981-99-8034-5_7
- Demchuk, M., Chubka, M., Pavliuk, B., Hroshovyi, T. (2022). Application of response surface methodology to optimize the technology of metformin oral dissolving tablets. Farmacia, 70 (1), 102–114. https://doi.org/10.31925/farmacia.2022.1.15
- Malanchuk, N. V., Demchuk, M. B., Hroshovyi, T. A. (2023). Study of the influence of excipients on the pharmaco-technological indicators of the tablets of bisoprolol fumarate and indapamide. Pharmaceutical Review, 4, 12–21. https://doi.org/10.11603/2312-0967.2023.4.14368
- Hroshovyi, T. A., Martsenyuk, V. P., Kucherenko, L. I., Vronska, L. V., Huryeyeva, C. M. (2008). Matematychne planuvannia eksperymentu pry provedenni naukovykh doslidzhen v farmatsii. Ternopil: TDMU; Ukrmedknyha, 368.
- Elyemni, M., Louaste, B., Ouadrhiri, F. E., Bouia, A., Eloutassi, N. (2021). Application of response surface methodology to optimize the extraction of essential oil from Rosmarinus officinalis using microwave-assisted hydrodistillation. Journal of Applied Pharmaceutical Science, 11 (1), 129–136. https://doi.org/10.7324/japs.2021.110115
- State Pharmacopoeia of Ukraine (2015). Kharkiv: State Enterprise “Ukrainian Scientific Pharmacopoeia Centre for the Quality of Medicines.”
- Butola, M., Badola, A., Nainwal, N., Rana, S., Jakhmola, V., Ale, Y., Ansori, A. N. M. (2024). Formulation of Sustained-Release Tablets of Tolperisone HCl Using Different Blends of Hydrophilic and Hydrophobic Polymers. Indian Journal of Pharmaceutical Education and Research, 58 (3s), s872–s879. https://doi.org/10.5530/ijper.58.3s.88
- Deepak Kumar, D., Ancheria, R., Shrivastava, S., Soni, S. L., Sharma, M. (2019). Review on Pharmaceutical Quality by Design (QbD). Asian Journal of Pharmaceutical Research and Development, 7 (2), 78–82. https://doi.org/10.22270/ajprd.v7i2.460
- Pavliuk, B., Chubka, M., Hroshovyi, T., Demchuk, M., Stechyshyn, I. (2022). The development of biodegradable hemostatic and absorbable sponges containing chlorhexidine digluconate and their in vitro characterization – A QbD approach. Journal of Applied Pharmaceutical Science, 12 (2), 56–65. https://doi.org/10.7324/JAPS.2021.120206
- Ayre, A. P., Gajbhiye, S. A. (2022). Formulation and Evaluation of Oro-dispersible Bromhexine Hydrochloride Granules Using Sachelac-80. International Journal of Pharmaceutical Investigation, 13 (1), 155–161. https://doi.org/10.5530/223097131733
- Alhamhoom, Y., Prakash, S. S., Kumar, A., Nanjappa, S. H., Rahamathulla, M., Kamath, M. S. et al. (2025). Formulation and Evaluation of Polymeric Spherical Agglomerates-Based Porous Orodispersible Tablets of Cilnidipine. Pharmaceutics, 17 (2), 170. https://doi.org/10.3390/pharmaceutics17020170
- Belayneh, A., Molla, F., Kahsay, G. (2020). Formulation and Optimization of Monolithic Fixed-Dose Combination of Metformin HCl and Glibenclamide Orodispersible Tablets. Advances in Pharmacological and Pharmaceutical Sciences, 2020, 1–14. https://doi.org/10.1155/2020/3546597
- Hemalatha, B., Ramu, A., Vidyadhara, S. (2025). Formulation and evaluation of azelnidipine fast-dissolving tablets. International Journal of Applied Pharmaceutics, 17 (1), 113–122. https://doi.org/10.22159/ijap.2025v17i1.52398
- PROSOLV® EASYtab SP. JRS Pharma. Retrieved from https://www.pharmaexcipients.com/product/prosolv-easytab-sp-lm/?attachment_id=190820&download_file=rfve7956ei1rz
- El-Nabarawi, M. A., Abd El-Monem, R. A., Al-Samadi, I. E. I. (2019). Effect of co-process excipients in formulation of ODTs using a model drug. International Journal of Pharmaceutical Sciences and Research, 10 (5), 2172–2181.
- Vlad, R.-A., Antonoaea, P., Todoran, N., Rédai, E.-M., Bîrsan, M., Muntean, D.-L. et al. (2022). Development and Evaluation of Cannabidiol Orodispersible Tablets Using a 23-Factorial Design. Pharmaceutics, 14 (7), 1467. https://doi.org/10.3390/pharmaceutics14071467
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Nadia Malanchuk, Demchuk Demchuk

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






