The effect of tablets containing dry extract of peony roots, L-tryptophan, and glycine on the condition of the brain in the context of experimental cranio-cerebral trauma
DOI:
https://doi.org/10.15587/2519-4852.2025.348668Keywords:
traumatic brain injury, neuroprotector, glycine, tryptophan, peony, neurons, enolase, S100 protein, rats, histologyAbstract
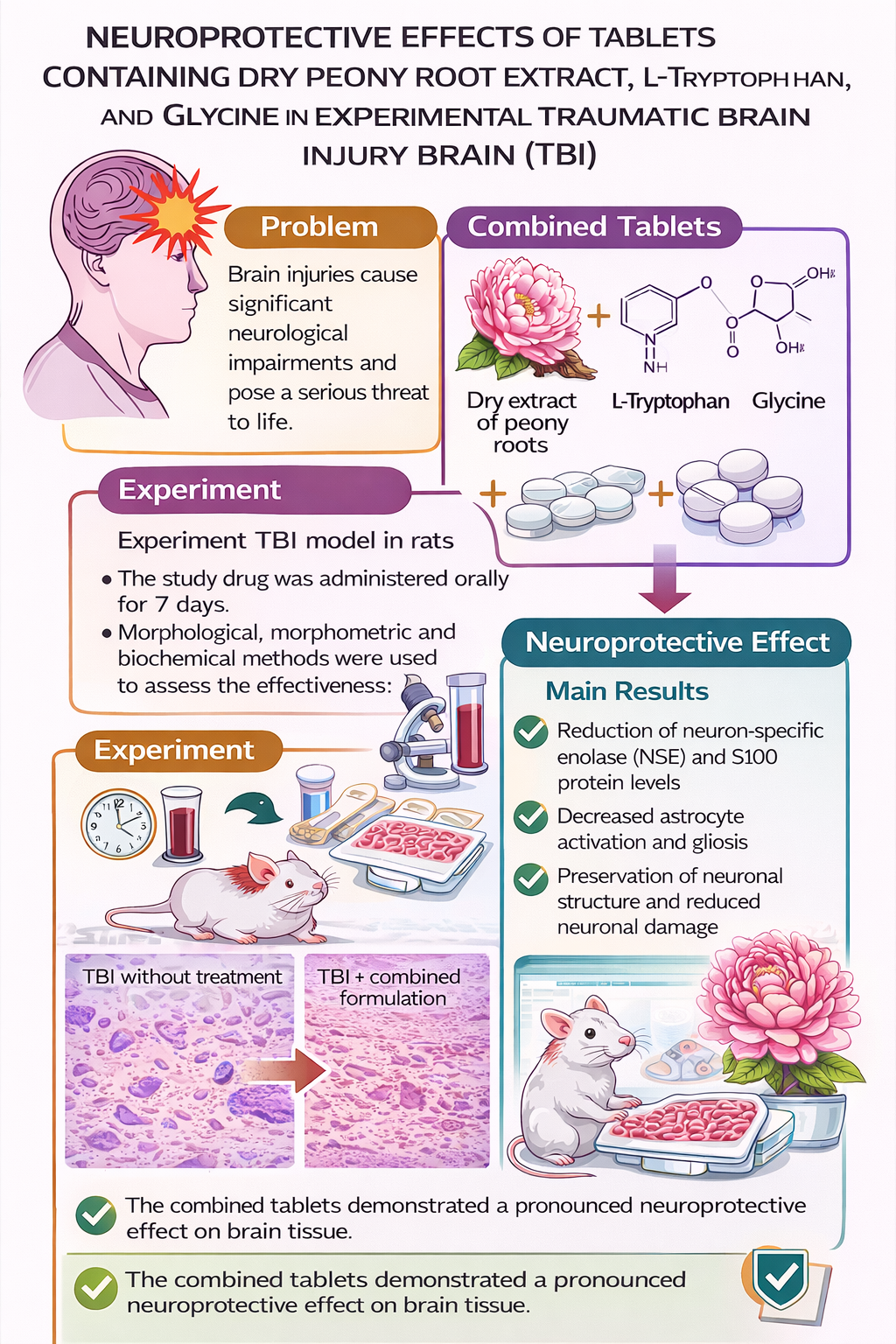
The aim of this work was to experimentally substantiate the neuroprotective potential of new combined tablets containing dry extract of peony roots, L-tryptophan, and glycine to eliminate the existing gap in the pharmacotherapy of traumatic brain injury (TBI) associated with the insufficient effectiveness of current monotherapeutic approaches.
Material and methods. The study was conducted on 40 male white rats weighing 200–250 g. The TBI model was induced by the free fall of a weight onto a fixed head of the animal. The test preparation was administered orally for 7 days. The effectiveness was assessed using morphological, morphometric, and biochemical methods, including the determination of neuron-specific enolase (NSE) and S100 protein levels in blood serum, as well as analysis of the condition of neurons in the sensorimotor cortex and hippocampus.
Results. Rats with TBI demonstrated a significant increase in NSE and S100 levels, a decrease in the number of normochromic neurons, activation of glial cells, and cerebral tissue oedema. Administration of the combined drug contributed to a reduction in neuronal damage markers, a decrease in the glio-neuronal index, and normalization of brain microstructures. Morphological examination revealed preservation of the neuronal layer and a reduction in destructive changes, indicating a pronounced neuroprotective effect of the drug.
Conclusions. The developed combined drug demonstrated an effective protective effect on brain tissue under conditions of experimental TBI. The obtained results substantiate the feasibility of further preclinical studies to investigate its mechanisms of action and potential clinical application
References
- Sariaslan, A., Sharp, D. J., D’Onofrio, B. M., Larsson, H., Fazel, S. (2016). Long-Term Outcomes Associated with Traumatic Brain Injury in Childhood and Adolescence: A Nationwide Swedish Cohort Study of a Wide Range of Medical and Social Outcomes. PLOS Medicine, 13 (8), e1002103. https://doi.org/10.1371/journal.pmed.1002103
- Dubinski, D., Kolesnyk, V. (2022). War in Ukraine: a neurosurgical perspective. Acta Neurochirurgica, 164 (12), 3071–3074. https://doi.org/10.1007/s00701-022-05388-3
- Schlager, P., Grgac, I., Herzer, G., Trimmel, H. (2025). Combined citicoline and Cerebrolysin for neuroprotection in traumatic brain injury: a retrospective cohort analysis. Frontiers in Neurology, 16. https://doi.org/10.3389/fneur.2025.1684981
- Best Practices Guidelines for the Management of Traumatic Brain Injury (2020). Chicago: American College of Surgeons, 148. Available at: https://www.facs.org/media/vgfgjpfk/best-practices-guidelines-traumatic-brain-injury.pdf
- Conti, F., McCue, J. J., DiTuro, P., Galpin, A. J., Wood, T. R. (2024). Mitigating Traumatic Brain Injury: A Narrative Review of Supplementation and Dietary Protocols. Nutrients, 16 (15), 2430. https://doi.org/10.3390/nu16152430
- Sato, K., Slobodin, T. M., Dziak, L. A., Hudz, I. M., Zaichenko, H. V., Zharinov, O. Y. et al. (2019). Neroprotektsiia: za chy proty. Zdorovia Ukrainy 21 storichchia, 4 (449), 5–7.
- Tang, H., Wu, L., Chen, X., Li, H., Huang, B., Huang, Z. et al. (2021). Paeoniflorin improves functional recovery through repressing neuroinflammation and facilitating neurogenesis in rat stroke model. PeerJ, 9, e10921. https://doi.org/10.7717/peerj.10921
- European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes (ETS No. 123) (1986). Strasbourg: Council of Europe. Available at: https://www.coe.int/en/web/conventions/full-list?module=treaty-detail&treatynum=123
- On the Protection of Animals from Brutal Treatment (2009). Law of Ukraine No. 1759-VI. 15.12.2009. Available at: https://zakon.rada.gov.ua/laws/show/3447-15
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes (2010). Official Journal of the European Union. Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32010L0063
- Khudoley, S., Ziablitsev, S. (2020). Experimental modeling of cholinoreactivity in traumatic brain injury: influence on central hemodynamic. Emergency Medicine, 16 (6), 110–115. https://doi.org/10.22141/2224-0586.16.6.2020.216519
- Chekman, I. S., Bielenichev, I. F., Nahorna, O. O., Horchakova, N. O., Lukianchuk, V. D., Bukhtiiarova, N. V. (2016). Doklinichne vyvchennia spetsyfichnoï aktyvnosti potetsiy̆nykh likarskykh zasobiv pervynnoï ta vtorynnoï ney̆roprotektsiï. Kyïv: TOV «Vydavnytstvo «Iuston», 93.
- Song, P., Yi, Z., Fu, Y., Song, D., Chen, K., Zheng, J. et al. (2021). Reversing Postcardiopulmonary Bypass Associated Cognitive Dysfunction Using k-Opioid Receptor Agonists to Regulate Microglial Polarization via the NLRP3/Caspase-1 Pathway. Journal of Healthcare Engineering, 2021, 1–12. https://doi.org/10.1155/2021/3048383
- Kural, A., Tekin Neijmann, Ş., Toker, A,, Doğan, H., Sever, N., Sarıkaya, S. (2020). Evaluation of rat major celluler prion protein for early diagnosis in experimental rat brain trauma model. Ulus Travma Acil Cerrahi Derg, 26 (1). https://doi.org/10.5505/tjtes.2018.46923
- Van De Werd, H. J. J. M., Rajkowska, G., Evers, P., Uylings, H. B. M. (2010). Cytoarchitectonic and chemoarchitectonic characterization of the prefrontal cortical areas in the mouse. Brain Structure and Function, 214 (4), 339–353. https://doi.org/10.1007/s00429-010-0247-z
- Kiernan, J. A. (2015). Histological and histochemical methods: theory and practice. Banbury: Scion Publishing, 571.
- Layton, C., Bancroft, J. D., Suvarna, S. K. (2019). Fixation of tissues. Bancroft’s Theory and Practice of Histological Techniques. St. Louis: Elsevier, 40–63. https://doi.org/10.1016/b978-0-7020-6864-5.00004-9
- Secades, J. J., Gareri, P. (2022). Citicoline: pharmacological and clinical review. 2022 update. Revue Neurologique, 75 (s05), 1–89. DOI: 10.33588/rn.75s05.2022311
- Bahraminejad, S., Almoazen, H. (2025). Sublingual and Buccal Delivery: A Historical and Scientific Prescriptive. Pharmaceutics, 17 (8), 1073. https://doi.org/10.3390/pharmaceutics17081073
- Onishi, H., Sakata, O. (2018). Absorption behavior of etilefrine after buccal administration in rats. International Journal of Pharmaceutics, 550 (1-2), 14–23. https://doi.org/10.1016/j.ijpharm.2018.08.009
- Tanaka, M., Szabó, Á., Vécsei, L. (2024). Redefining Roles: A Paradigm Shift in Tryptophan–Kynurenine Metabolism for Innovative Clinical Applications. International Journal of Molecular Sciences, 25 (23), 12767. https://doi.org/10.3390/ijms252312767
- Huang, Y., Zhao, M., Chen, X., Zhang, R., Le, A., Hong, M. et al. (2023). Tryptophan Metabolism in Central Nervous System Diseases: Pathophysiology and Potential Therapeutic Strategies. Aging and Disease, 14 (3), 858–878. https://doi.org/10.14336/ad.2022.0916
- Sun, Y., Wang, S., Liu, B., Hu, W., Zhu, Y. (2023). Host-Microbiome Interactions: Tryptophan Metabolism and Aromatic Hydrocarbon Receptors after Traumatic Brain Injury. International Journal of Molecular Sciences, 24 (13), 10820. https://doi.org/10.3390/ijms241310820
- Soh, J., Raventhiran, S., Lee, J. H., Lim, Z. X., Goh, J., Kennedy, B. K., Maier, A. B. (2023). The effect of glycine administration on the characteristics of physiological systems in human adults: A systematic review. GeroScience, 46 (1), 219–239. https://doi.org/10.1007/s11357-023-00970-8
- Zahra, N., Iqbal, J., Arif, M., Abbasi, B. A., Sher, H., Nawaz, A. F. et al. (2023). A comprehensive review on traditional uses, phytochemistry and pharmacological properties of Paeonia emodi Wall. ex Royle: current landscape and future perspectives. Chinese Medicine, 18 (1). https://doi.org/10.1186/s13020-023-00727-7
- Wiegand, V., Gao, Y., Teusch, N. (2024). Pharmacological Effects of Paeonia lactiflora Focusing on Painful Diabetic Neuropathy. Planta Medica, 90 (15), 1115–1129. https://doi.org/10.1055/a-2441-6488
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Nadiia Kononenko, Ruslan Mirzaliiev

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






