Analysis of state macroeconomic indicators of healthcare systems development in reference countries for the domestic pharmaceutical market
DOI:
https://doi.org/10.15587/2519-4852.2025.349045Keywords:
accessibility of medicines, external reference pricing, regulation of drug prices, healthcare system, pharmaceutical provision of the population, pharmaceutical marketAbstract
Regulation of drug availability is one of the most important areas of state policy in the pharmaceutical market. The use of various methods of price regulation allows achieving the desired socio-economic results, but the complexity of the processes taking place in the pharmaceutical market and in the state necessitates the constant revision of existing approaches and measures.
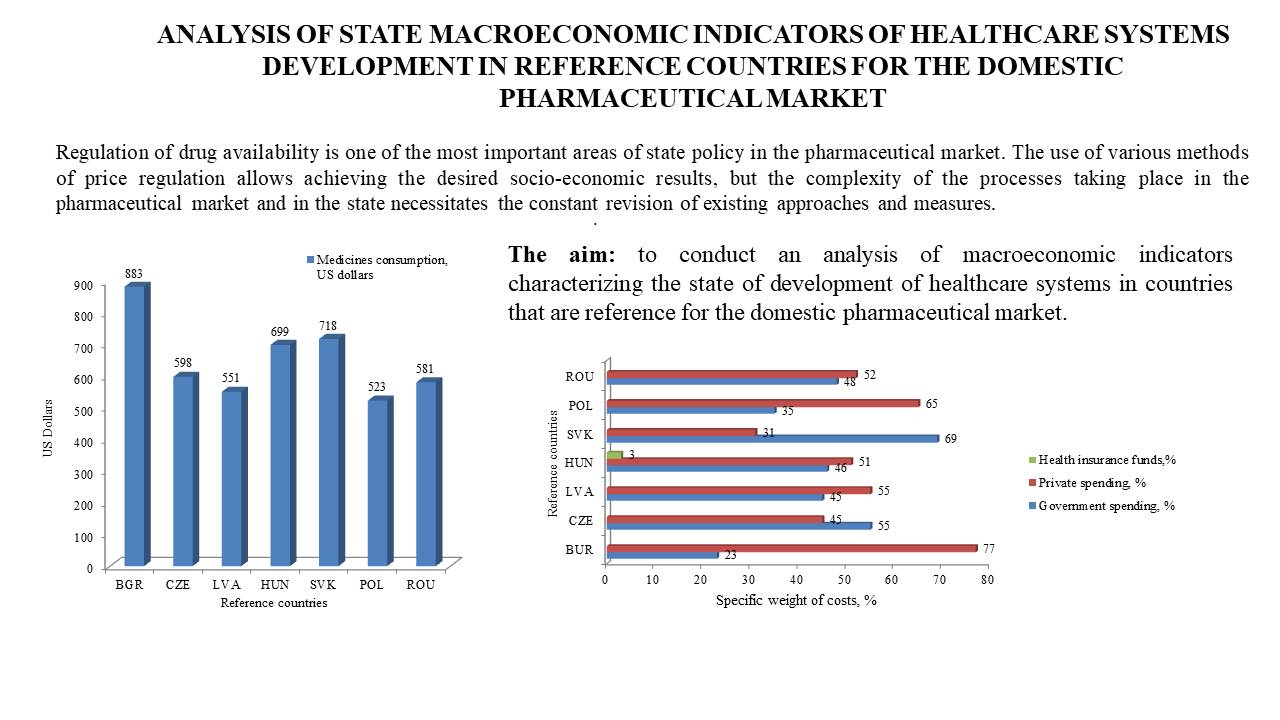
The aim: to conduct an analysis of macroeconomic indicators characterizing the state of development of healthcare systems in countries that are reference for the domestic pharmaceutical market.
Materials and methods. General theoretical (historical, formal, graphical, hypothetical-deductive, etc.) and applied (organizational-economic, mathematical-statistical, etc.) research methods were used. The subject of the research was the World Bank health indicators, which are integrated with WHO data, and data from the Organization for Economic Cooperation and Development for reference countries (Poland, Slovakia, the Czech Republic, Latvia, Hungary, Romania, Moldova, Bulgaria) and in Ukraine.
Results. It was found that for the vast majority of indicators characterizing the state of health care financing, domestic indicators significantly differed from the average values for the group of reference countries. For indicators of domestic health care expenditures (four indicators), domestic data had critically low values (52.12% –% in current healthcare spending, expenditures per capita, including at purchasing power parity of the population – $ 192.81 and $ 570.60, respectively). Only for the indicator of domestic public health care expenditures as a% of general government expenditures, Ukrainian indicators (10.56%) were within the fluctuation range of the corresponding data for the group of reference countries. A comparative analysis of current healthcare expenditures (3 indicators) showed that out of the three indicators, the values of two in Ukraine were close to the minimum values in the group of reference countries. These are current healthcare expenditures per capita, including at purchasing power parity. In Ukraine, the values of these indicators were $369.90 and $1095.06, which were 3.72 and 2.49 times lower than the average values for the group of reference countries. According to the indicators characterizing the participation of private capital and citizens in healthcare expenditures (5 indicators), it was established that per capita private expenditures in Ukraine were $177.10, which was 1.88 times lower than the average for the group of reference countries ($332.58). In terms of private and out-of-pocket expenditures as a% of current healthcare expenditures, domestic data had the highest values compared to reference countries. Thus, private and out-of-pocket expenditures in Ukraine were 1.76 and 1.88 times higher than the average values for reference countries (27.07% and 24.04%, respectively). Domestic out-of-pocket expenditures calculated per capita ($167.54) and at purchasing power parity ($ 495.88) were 44,49% and 17,51% lower than the corresponding average values for the group of reference countries ($301.82 and $ 601.10, respectively). It was found that within the group of reference countries, the drug consumption index varied. This index ranged from $523.0 (Poland) to $883.0 (Bulgaria). The average consumption value in Ukraine for 2021-2023 was ($112.33), which was 5.79 times less than the corresponding data for the group of reference countries. In addition, in the group of reference countries, there are fundamental differences in the structure of population spending on medicines by funding sources (state, private revenues, and health insurance funds).
Conclusions. Significant differences in macroeconomic indicators characterizing the state of healthcare financing in the reference countries and in Ukraine have been identified. This necessitates further research on the topic and a review of the group of reference countries used in the domestic pharmaceutical market to regulate the socio-economic accessibility of medicines for the population
References
- Gautier, L., David, P.-M. (2022). “Health for all” and the challenges for pharmaceutical policies: A critical interpretive synthesis over 40 years. Social Sciences & Humanities Open, 5 (1), 100255. https://doi.org/10.1016/j.ssaho.2022.100255
- Hill, S. R. (2015). Affordable innovation: future directions in pharmaceutical policy. Journal of Pharmaceutical Policy and Practice, 8 (S1). https://doi.org/10.1186/2052-3211-8-s1-k1
- Lilja, J., Salek, S., Alvarez, A., Hamilton, D. (2008). Pharmaceutical Systems. Global Perspectives. John Wiley & Sons Ltd., 359.
- Ocran Mattila, P., Ahmad, R., Hasan, S. S., Babar, Z.-U.-D. (2021). Availability, Affordability, Access, and Pricing of Anti-cancer Medicines in Low- and Middle-Income Countries: A Systematic Review of Literature. Frontiers in Public Health, 9. https://doi.org/10.3389/fpubh.2021.628744
- Fundytus, A., Sengar, M., Lombe, D., Hopman, W., Jalink, M., Gyawali, B. et al. (2021). Access to cancer medicines deemed essential by oncologists in 82 countries: an international, cross-sectional survey. The Lancet Oncology, 22 (10), 1367–1377. https://doi.org/10.1016/s1470-2045(21)00463-0
- Patel, S. S., Erickson, T. B. (2022). The New Humanitarian Crisis in Ukraine: Coping With the Public Health Impact of Hybrid Warfare, Mass Migration, and Mental Health Trauma. Disaster Medicine and Public Health Preparedness, 16 (6), 2231–2232. https://doi.org/10.1017/dmp.2022.70
- Habicht, J., Hellowell, M., Kutzin, J. (2024). Sustaining progress towards universal health coverage amidst a full-scale war: learning from Ukraine. Health Policy and Planning, 39 (7), 799–802. https://doi.org/10.1093/heapol/czae041
- Deiaki pytannia derzhavnoho rehuliuvannia tsin na likarski zasoby (2025). Postanova KMU No. 439. 04.04.2025. Available at: https://zakon.rada.gov.ua/laws/show/439-2025-%D0%BF#Text Last accessed: 09.11.2025
- Pro vnesennia zmin do poriadkiv, zatverdzhenykh postanovoiu Kabinetu Ministriv Ukrainy vid 4 kvitnia 2025 roku No. 439 (2025). Postanova KMU No. 1013. 20.08.2025. Available at: https://zakon.rada.gov.ua/laws/show/1013-2025-%D0%BF#Text Last accessed: 09.11.2025
- Protocol Development for Systematic Reviews (2024). Veritas Health Innovation Ltd. Available at: https://www.covidence.org/wp-content/uploads/2024/10/A_practical_guide_Protocol_Development_for_Systematic_Reviews.pdf Last accessed: 08.11.2025
- Іndicator. World Bank Group. Available at: https://data.worldbank.org/indicator Last accessed: 02.12.2025
- Samborskyi, O., Panfilova, Н., Baihush, Y., Simonian, L., Bilyk, I., Martyniuk, T. et al. (2022). Comparative analysis of pharmaceutical supply systems of the population of European countries according to a complex of socio-economic indicators. ScienceRise: Pharmaceutical Science, 5 (39), 16–28. https://doi.org/10.15587/2519-4852.2022.265814
- Kotvitska, A., Volkova, A., Korzh, I., Surikova, I. (2021). Comparative analysis of indicators that determine the effectiveness of the implementation of socio-economic determinants of health in Europe and Ukraine. ScienceRise: Pharmaceutical Science, 3 (31), 34–41. https://doi.org/10.15587/2519-4852.2021.235787
- Health at a Glance 2025: OECD Indicators (2025). Paris: OECD Publishing. https://doi.org/10.1787/8f9e3f98-en
- Dixon-Woods, M. (2006). Critical interpretive synthesis: What it is and why it is needed Chochrane colloquium abstracts journal. Dublin. Available at: https://abstracts.cochrane.org/2006-dublin/critical-interpretive-synthesis-what-it-and-why-it-needed Last accessed: 22.11.2025
- Pita Barros, P. (2010). Pharmaceutical policies in European countries. Advanced Health Economical Health Service Research. Pharmaceutical Markets and Insurance Worldwide. Emerald Group Publishing Limited, 3–27. https://doi.org/10.1108/s0731-2199(2010)0000022004
- Jacobzone, S. (2000). Pharmaceutical Policies in OECD Countries: Reconciling Social and Industrial Goals». OECD Labour Market and Social Policy Occasional Papers No. 40. Available at: https://doi.org/10.1787/323807375536
- Infohrafichne doslidzhennia. Farmatsevtychna haluz Ukrainy 2023 r. (2024). PROXIMA. Available at: https://proximaresearch.com/ua/ua/novini/ua-pharma-industry-2023-by-proxima-research/ Last accessed: 15.11.2025
- Malinowski, K. P., Kawalec, P., Trąbka, W., Sowada, C., Petrova, G., Manova, M. et al. (2020). Health technology assessment and reimbursement policy for oncology orphan drugs in Central and Eastern Europe. Orphanet Journal of Rare Diseases, 15 (1). https://doi.org/10.1186/s13023-020-01556-9
- Serrano Valera, M., Martínez-Alcalá, I., Piuvezam, G., Mateo-Ramírez, F., Pimenta, I. D. S. F., Vela, N. (2024). Pharmaceutical products, drugs and personal care products in European waters: A protocol for systematic review and meta-analysis. PLOS ONE, 19 (8), e0308975. https://doi.org/10.1371/journal.pone.0308975
- Kanavos, P., Fontrier, A. -M., Gill, J., Kyriopoulos, D. (2017). The Implementation of External Reference Pricing within and across Country Borders. 2017. London School of Economics. https://doi.org/10.21953/lse.y1tbizsxrl3n
- Kanavos, P. G., Tzouma, V., Fontrier, A.-M., Kamphuis, B., Parkin Colville, G., Saleh, S. (2018). Pharmaceutical pricing and reimbursement in the Middle East and North Africa region. The London school of economics and political science. Available at: https://www.lse.ac.uk/business/consulting/assets/documents/the-implementation-of-external-reference-pricing-within-and-across-country-borders.pdf Last accessed: 02.11.2025
- Exploring the feasibility of sharing information on medicine prices across countries (2024). OECD Health Working Papers No. 171. https://doi.org/10.1787/5e4a7a47-en
- Batt, S. (2016). Pharmaceutical Company Corruption and the Moral Crisis in Medicine. Hastings Center Report, 46 (4), 10–13. https://doi.org/10.1002/hast.575
- Pezzola, A., Sweet, C. M. (2016). Global pharmaceutical regulation: the challenge of integration for developing states. Globalization and Health, 12 (1). https://doi.org/10.1186/s12992-016-0208-2
- Lakdawalla, D. N. (2018). Economics of the Pharmaceutical Industry. Journal of Economic Literature, 56 (2), 397–449. https://doi.org/10.1257/jel.20161327
- Lee, I.-H., Bloor, K., Hewitt, C., Maynard, A. (2014). International experience in controlling pharmaceutical expenditure: influencing patients and providers and regulating industry – a systematic review. Journal of Health Services Research & Policy, 20 (1), 52–59. https://doi.org/10.1177/1355819614545675
- Post, H. C., Schutte, T., van Oijen, M. G. H., van Laarhoven, H. W. M., Hollak, C. E. M. (2023). Time to reimbursement of novel anticancer drugs in Europe: a case study of seven European countries. ESMO Open, 8 (2), 101208. https://doi.org/10.1016/j.esmoop.2023.101208
- Espin, J., Schlander, M., Godman, B., Anderson, P., Mestre-Ferrandiz, J., Borget, I. et al. (2018). Projecting Pharmaceutical Expenditure in EU5 to 2021: Adjusting for the Impact of Discounts and Rebates. Applied Health Economics and Health Policy, 16(6), 803–817. https://doi.org/10.1007/s40258-018-0419-1
- Kucherenko, L. I., Nizhenkovska, I. V., Sholoiko, N. V., Hala, L. O., Datsiuk, N. O. (2023). External reference pricing for medicines in Ukraine: latest trends. Current Issues in Pharmacy and Medicine: Science and Practice, 16 (3), 272–276. https://doi.org/10.14739/2409-2932.2023.3.287758
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Hanna Panfilova, Marta Matushchak, Oleh Samborskyi, Alina Volkova, Liubov Tereshchenko, Yuliia Baihush, Liusine Simonian, Tetiana Martyniuk, Halyna Tsikhon

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






