Obtaining the substance enoxaparin sodium equivalent to the original Clexane® and Lovenox®. Selection of technological parameters of the key stage of the synthesis
DOI:
https://doi.org/10.15587/2519-4852.2023.277735Keywords:
Enoxaparin, Low molecular weight heparin, technological parameters, compositional analysis, HSQC, size-exclusion chromatography, reducing, non-reducing endsAbstract
The aim: to carry out the key stage of synthesis to obtain a substance equivalent to the original drugs Clexane® and Lovenox® by determining the technological parameters of the synthesis that are critical from the point of view of the formation of the molecule and studying the correlation between the structural characteristics of Enoxaparin samples and the experimental conditions of the technological process.
Materials and methods: samples of the Enoxaparin sodium substance were synthesized according to the method described in the patent, as well as with a variation of the selected critical technological parameters. The obtained samples of Enoxaparin sodium were analyzed according to pharmacopoeial requirements, as well as by non-pharmacopoeial methods, such as two-dimensional NMR spectroscopy and size exclusion chromatography for detailed structural characterization of the molecule.
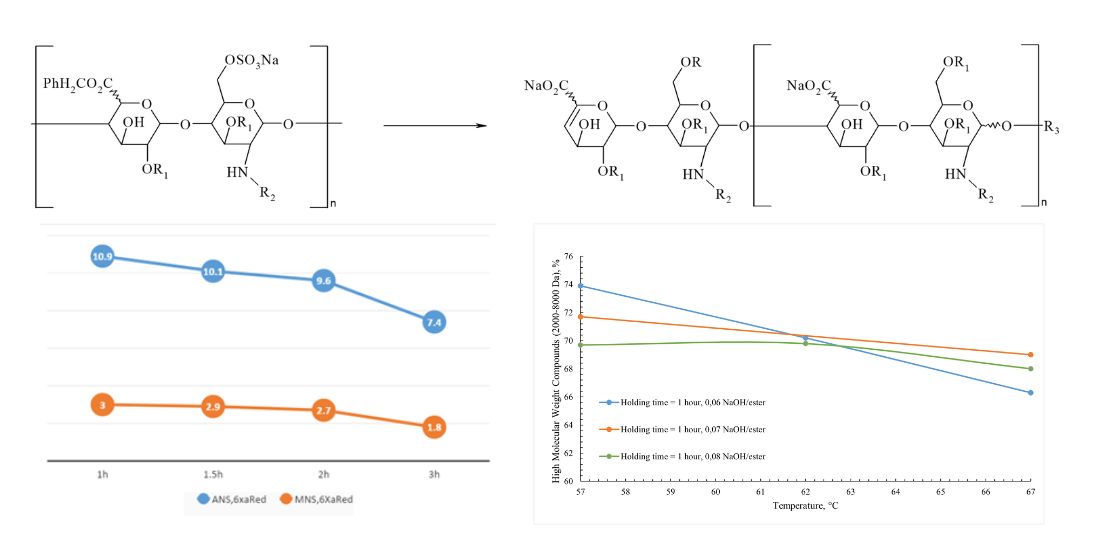
Results: determination and variation of technological parameters critical for the formation of the molecule, such as temperature, the amount of alkali for the depolymerization reaction, and the reaction time of the reaction mass, were determined and varied. Enoxaparin sodium samples were developed according to the selected parameters and a detailed analysis of the structure of the obtained samples was carried out, followed by a comparison with the original Clexane® and Lovenox®. It was established that with an increase in the temperature of the reaction mass, the amount of alkali and the holding time individually and in combination, the degree of depolymerization increases, which makes the composition of the molecule unbalanced in comparison with the original drugs Clexane® and Lovenox®.
Conclusions: As a result of the experiments, the technological parameters of the synthesis of a sample of Enoxaparin sodium were evaluated and determined, allowing to obtain a substance comparable to the originator in terms of chemical structure (alkali/ heparin benzyl ester ratio 0.06; temperature – 57 °C, reaction mixture holding time - 1.5 hours)
Supporting Agency
- JSC Farmak
References
- Taylor, A., Martinez-Quinones, P., Huang, E., Robinson, T., White, C. Q. (2022). Effective use of weight-based enoxaparin for deep vein thrombosis chemoprophylaxis in patients with traumatic brain injury. The American Journal of Surgery, 223 (1), 146–150. doi: https://doi.org/10.1016/j.amjsurg.2021.07.030
- Billett, H. H., Reyes-Gil, M., Szymanski, J., Ikemura, K., Stahl, L. R., Lo, Y. et al. (2020). Anticoagulation in COVID-19: Effect of Enoxaparin, Heparin, and Apixaban on Mortality. Thrombosis and Haemostasis, 120 (12), 1691–1699. doi: https://doi.org/10.1055/s-0040-1720978
- Drago, F., Gozzo, L., Li, L., Stella, A., Cosmi, B. (2020). Use of Enoxaparin to Counteract COVID-19 Infection and Reduce Thromboembolic Venous Complications: A Review of the Current Evidence. Frontiers in Pharmacology, 11. doi: https://doi.org/10.3389/fphar.2020.579886
- Casu, B. (2005). Structure and Active Domains of Heparin. Chemistry and Biology of Heparin and Heparan Sulfate, 1–28. doi: https://doi.org/10.1016/b978-008044859-6/50002-2
- Alekseeva, A., Elli, S., Cosentino, C., Torri, G., Naggi, A. (2014). Susceptibility of enoxaparin reducing end amino sugars to periodate oxidation. Carbohydrate Research, 400, 33–43. doi: https://doi.org/10.1016/j.carres.2014.08.016
- Mourier, P. A. J., Agut, C., Souaifi-Amara, H., Herman, F., Viskov, C. (2015). Analytical and statistical comparability of generic enoxaparins from the US market with the originator product. Journal of Pharmaceutical and Biomedical Analysis, 115, 431–442. doi: https://doi.org/10.1016/j.jpba.2015.07.038
- Weitz, J. I. (1997). Low-Molecular-Weight Heparins. New England Journal of Medicine, 337 (10), 688–698. doi: https://doi.org/10.1056/nejm199709043371007
- Langeslay, D. J., Beecher, C. N., Dinges, M. M., Larive, C. K. (2013). Glycosaminoglycan Structural Characterization. EMagRes. doi: https://doi.org/10.1002/9780470034590.emrstm1316
- Wang, T., Liu, L., Voglmeir, J. (2020). Chemoenzymatic synthesis of ultralow and low-molecular weight heparins. Biochimica et Biophysica Acta (BBA) – Proteins and Proteomics, 1868 (2), 140301. doi: https://doi.org/10.1016/j.bbapap.2019.140301
- Mourier, P. A. J., Herman, F., Sizun, P., Viskov, C. (2016). Analytical comparison of a US generic enoxaparin with the originator product: The focus on comparative assessment of antithrombin-binding components. Journal of Pharmaceutical and Biomedical Analysis, 129, 542–550. doi: https://doi.org/10.1016/j.jpba.2016.07.033
- Iqbal, Z., Sadaf, S. (2022). Commercial Low Molecular Weight Heparins – Patent Ecosystem and Technology Paradigm for Quality Characterization. Journal of Pharmaceutical Innovation. doi: https://doi.org/10.1007/s12247-022-09665-7
- Information on Adverse Event Reports and Heparin. Available at: http://wayback.archive-it.org/7993/20161024045926/http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm112669.htm
- Shriver, Z., Sasisekharan, R. (2009). From crisis to opportunity: A perspective on the heparin crisis. Thrombosis and Haemostasis, 102 (11), 854–858. doi: https://doi.org/10.1160/th09-02-0083
- Guerrini, M., Beccati, D., Shriver, Z., Naggi, A., Viswanathan, K., Bisio, A. et al. (2008). Oversulfated chondroitin sulfate is a contaminant in heparin associated with adverse clinical events. Nature Biotechnology, 26 (6), 669–675. doi: https://doi.org/10.1038/nbt1407
- Szajek, A. Y., Chess, E., Johansen, K., Gratzl, G., Gray, E., Keire, D. et al. (2016). The US regulatory and pharmacopeia response to the global heparin contamination crisis. Nature Biotechnology, 34 (6), 625–630. doi: https://doi.org/10.1038/nbt.3606
- Ye, H., Toby, T. K., Sommers, C. D., Ghasriani, H., Trehy, M. L., Ye, W. et al. (2013). Characterization of currently marketed heparin products: Key tests for LMWH quality assurance. Journal of Pharmaceutical and Biomedical Analysis, 85, 99–107. doi: https://doi.org/10.1016/j.jpba.2013.06.033
- Guideline on non-clinical and clinical development of similar biological medicinal products containing lowmolecular-weight-heparins (2016). Available at: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-non-clinical-clinical-development-similar-biological-medicinal-products-containing-low_en.pdf
- ImmunogenicityRelated Considerations for Low Molecular Weight Heparin (2016). Pharmaceutical Quality/CMC. Available at: https://www.fda.gov/files/drugs/published/Immunogenicity-Related-Considerations-for-Low-Molecular-Weight-Heparin-Guidance-for-Industry.pdf
- Ofosu, F. A. (2010). The United States Food and Drugs Administration Approves a Generic Enoxaparin. Clinical and Applied Thrombosis/Hemostasis, 17 (1), 5–8. doi: https://doi.org/10.1177/1076029610389028
- Guerrini, M., Elli, S., Gaudesi, D., Torri, G., Casu, B., Mourier, P. et al. (2010). Effects on Molecular Conformation and Anticoagulant Activities of 1,6-Anhydrosugars at the Reducing Terminal of Antithrombin-Binding Octasaccharides Isolated from Low-Molecular-Weight Heparin Enoxaparin. Journal of Medicinal Chemistry, 53 (22), 8030–8040. doi: https://doi.org/10.1021/jm100771s
- Guan, Y., Xu, X., Liu, X., Sheng, A., Jin, L., Linhardt, R. J., Chi, L. (2016). Comparison of Low-Molecular-Weight Heparins Prepared From Bovine Lung Heparin and Porcine Intestine Heparin. Journal of Pharmaceutical Sciences, 105 (6), 1843–1850. doi: https://doi.org/10.1016/j.xphs.2016.03.037
- Debrie, R. (1995). Pat. US5389618A. Mixtures of particular LMW heparinic polysaccharides for the prophylaxis/treatment of acute thrombotic events. published: 14.02.1995.
- Adiguzel, C., Jeske, W. P., Hoppensteadt, D., Walenga, J. M., Bansal, V., Fareed, J. (2009). Structural and Functional Characterization of Low-molecular-weight Heparins: Impact on the Development of Guidelines for Generic Products. Clinical and Applied Thrombosis/Hemostasis, 15 (2), 137–144. doi: https://doi.org/10.1177/1076029609332727
- Arnold, K., Capuzzi, S., Xu, Y., Muratov, E., Carrick, K., Szajek, A. et al. (2017). Modernization of Enoxaparin Molecular Weight Determination Using Homogeneous Standards. Pharmaceuticals, 10 (3), 66. doi: https://doi.org/10.3390/ph10030066
- Wanisa, A. M., Qasem, A. A., Asma, O. E. (2020). Green chemistry: principles, applications, and disadvantages. Chemical Methodologies, 4 (4), 408–423. doi: https://doi.org/10.33945/sami/chemm.2020.4.4
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Yuliia Bovsunovska, Vitalii Rudiuk, Volodymyr Mishchenko, Victoriya Georgiyants

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.







