Comparative analysis of pharmaceutical supply systems of the population of European countries according to a complex of socio-economic indicators
DOI:
https://doi.org/10.15587/2519-4852.2022.265814Keywords:
drug costs, health care costs, reimbursement of drugs, the system of pharmaceutical provision of the population, the pharmaceutical marketAbstract
The aim: to conduct an analysis of the state of functioning of pharmaceutical supply systems for the population in European countries and Ukraine based on a set of socio-economic indicators.
Materials and methods. General theoretical (historical, formal, graphic, hypothetical-deductive) and applied (organizational-economic, mathematical-statistical) research methods were used. The object of research was data that was freely available.
Results. It was established that the highest volume of the pharmaceutical market volume per inhabitant is typical for Italy (410.18 euros) and the lowest for Ukraine (53.58 euros). It has been proven that with an increase in GDP indicators, which are calculated based on purchasing power parity (PPP) per capita, the range of variation of this indicator by groups decreases, and the number of countries with a negative value of the foreign trade balance also decreases. Within groups of countries, there is a different level of dependence on the import of pharmaceutical products. In Ukraine (the first group), imports exceeded the export data of pharmaceutical products by 8.6 times. For other countries in this group (Latvia, Bulgaria, and Slovakia), imports exceeded exports by 1.3, 1.4, and 4.0 times, respectively. The highest values of the volume of foreign trade in pharmaceutical products were characteristic of the countries of the third and second groups. The undisputed leader is Germany (134,541.0 million euros), in second place is Italy (59,533.0 million euros), and in third place is France (58,568.0 million euros).
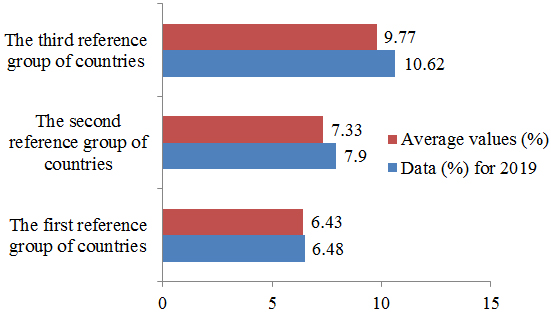
The highest values of health care costs as a percentage of GDP (%) are typical for the countries of the third group and the lowest for the countries of the first group. For all countries, this indicator had a characteristic tendency to increase over time. Growth rates varied both across groups and within groups across countries. According to the indicator of the amount of reimbursement of the cost of drug consumption per person, there was a significant fluctuation of the data by country within the groups. The most extensive range of fluctuations was observed in the third group (284.83 euros). The highest reimbursement amounts are typical for Germany (483.53 euros) and the lowest for Bulgaria (54.25 euros). In most countries, there is a high level (50.0 % and higher) of state participation in the payment of medicine, except for Poland (36.0 %), Lithuania (34.0 %) and Latvia (37.0 %). The lowest values of money consumers spend to pay for medicines (from 13.0 % to 44.0 %) are characteristic of the countries of the third group.
Conclusions. The established peculiarities of the functioning of the pharmaceutical supply systems of European countries should not diminish the value of the state's aspirations to harmonize the processes that take place to promote medicinal products to consumers
References
- Lakdawalla, D. N. (2018). Economics of the Pharmaceutical Industry. Journal of Economic Literature, 56 (2), 397–449. doi: https://doi.org/10.1257/jel.20161327
- Pezzola, A., Sweet, C. M. (2016). Global pharmaceutical regulation: the challenge of integration for developing states. Globalization and Health, 12 (1). doi: https://doi.org/10.1186/s12992-016-0208-2
- Rogers, D., Rogers, B., Lewis, J., Lewis, E. (2018). The UK pharmaceutical in¬dustry braces for Brexit, be it mild, severe, or doomsday. Medical Writing, 27 (4), 41–45.
- Espin, J., Schlander, M., Godman, B., Anderson, P., Mestre-Ferrandiz, J., Borget, I. et. al. (2018). Projecting Pharmaceutical Expenditure in EU5 to 2021: Adjusting for the Impact of Discounts and Rebates. Applied Health Economics and Health Policy, 16 (6), 803–817. doi: https://doi.org/10.1007/s40258-018-0419-1
- Drummond, M., Towse, A. (2019). Is rate of return pricing a useful approach when value-based pricing is not appropriate? The European Journal of Health Economics, 20 (7), 945–948. doi: https://doi.org/10.1007/s10198-019-01032-7
- Batt, S. (2016). Pharmaceutical Company Corruption and the Moral Crisis in Medicine. Hastings Center Report, 46 (4), 10–13. doi: https://doi.org/10.1002/hast.575
- Eger, S., Mahlich, J. C. (2014). Pharmaceutical regulation in Europe and its impact on corporate R&D. Health Economics Review, 4 (1). doi: https://doi.org/10.1186/s13561-014-0023-5
- Shaikh, M., Del Giudice, P., Kourouklis, D. (2020). Revisiting the Relationship Between Price Regulation and Pharmaceutical R&D Investment. Applied Health Economics and Health Policy, 19 (2), 217–229. doi: https://doi.org/10.1007/s40258-020-00601-9
- Panteli, D., Arickx, F., Cleemput, I., Dedet, G., Eckhardt, H., Fogarty, E., Kaitelidou, D. (2016). Pharmaceutical regulation in 15 European countries. Health Systems in Transition, 18 (5), 1–118.
- Maynard, A., Bloor, K. (2015). Regulation of the pharmaceutical industry: promoting health or protecting wealth? Journal of the Royal Society of Medicine, 108 (6), 220–222. doi: https://doi.org/10.1177/0141076814568299
- Hawkes, N. (2014). Cancer Drugs Fund receives boost but will no longer fund “overpriced” drugs. BMJ, 349 (sep01 8), g5382–g5382. doi: https://doi.org/10.1136/bmj.g5382
- Mestre-Ferrandiz, J., Palaska, C., Kelly, T., Hutchings, A., Parnaby, A. (2019). An analysis of orphan medicine expenditure in Europe: is it sustainable? Orphanet Journal of Rare Diseases, 14 (1). doi: https://doi.org/10.1186/s13023-019-1246-7
- Urbinati, D., Rémuzat, C., Kornfeld, Å., Vataire, A.-L., Cetinsoy, L., Aballéa, S., Mzoughi, O., Toumi, M. (2014). EU pharmaceutical expenditure forecast. Journal of Market Access & Health Policy, 2 (1), 23738. doi: https://doi.org/10.3402/jmahp.v2.23738
- Lee, I.-H., Bloor, K., Hewitt, C., Maynard, A. (2014). International experience in controlling pharmaceutical expenditure: influencing patients and providers and regulating industry – a systematic review. Journal of Health Services Research & Policy, 20 (1), 52–59. doi: https://doi.org/10.1177/1355819614545675
- Сommunication from the commission to the European Parliament, the council, the European economic and social committee and the committee of the regions Pharmaceutical Strategy for Europea. Pudlic Health. European Commission. Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52020DC0761
- Piachaud-Moustakis, B. (2022). The European Union’s New Pharmaceutical Strategy for Europe. Pharmaceutical Technology, Pharmaceutical Technology, 46 (7), 7–8. Available at: https://www.pharmtech.com/view/the-european-union-s-new-pharmaceutical-strategy-for-europe
- Pinto, C. M., Roy, F. (2021). Revision of the EU General Pharmaceuticals Legislation – Public Consultation is now Open. Hogan Lovells.
- Shaikh, M., Del Giudice, P., Kourouklis, D. (2020). Revisiting the Relationship Between Price Regulation and Pharmaceutical R&D Investment. Applied Health Economics and Health Policy, 19 (2), 217–229. doi: https://doi.org/10.1007/s40258-020-00601-9
- Godman, B., Fadare, J., Kwon, H.-Y., Dias, C. Z., Kurdi, A., Dias Godói, I. P. et/ al. (2021). Evidence-based public policy making for medicines across countries: findings and implications for the future. Journal of Comparative Effectiveness Research, 10 (12), 1019–1052. doi: https://doi.org/10.2217/cer-2020-0273
- GDP per capita, PPP (current international $). The World Bank. Available at: https://data.worldbank.org/indicator/NY.GDP.PCAP.PP.CD
- Fang, J.-Q. (Ed.) (2017). Handbook of Medical Statistics. Sun Yat-Sen University.
- Pro vnesennia zmin do Poriadku rozrakhunku hranychnykh optovo-vidpusknykh tsin na likarski zasoby, yaki vyznacheni u pereliku likarskykh zasobiv, shcho vkliucheni do Natsionalnoho pereliku osnovnykh likarskykh zasobiv ta na yaki vstanovliuiutsia hranychni optovo-vidpuskni tsiny (2020). Nakaz MOZ Ukrainy No. 139. 22.01.2020. Available at: https://zakon.rada.gov.ua/laws/show/z0133-20#Text
- The Pharmaceutical Industry in Figures (2021). International Federation of Pharmaceutical Manufacturers and Associations. Available at: https://www.efpia.eu/media/602709/the-pharmaceutical-industry-in-figures-2021.pdf
- The pharmaceutical industry and Global health facts and figures 2021 (2021). International Federation of Pharmaceutical Manufacturers and Associations, 102. Available at: https://www.ifpma.org/wp-content/uploads/2021/04/IFPMA-Facts-And-Figures-2021.pdf
- Rashidian, A., Soleymani, F., Cheraghali, A., Kebriaeezade, A., Kheirandish, M. (2015). A review of pharmaceutical policies in response to economic crises and sanctions. Journal of Research in Pharmacy Practice, 4 (3), 115–122. doi: https://doi.org/10.4103/2279-042x.162361
- Barfoed, C. (2016). The Attractiveness of the European pharmaceutical market and its explanatory factors. Copenhagen Business School, 135. Available at: https://research-api.cbs.dk/ws/portalfiles/portal/58432276/camilla_barfoed.pdf
- Mazaraki, A. A. (2014). Mizhnarodna ekonomika. Part 1. Kyiv: Kyiv. nats. torh.-ekon. un-t, 564.
- Health at a Glance 2019 (2019). Health at a Glance. doi: https://doi.org/10.1787/4dd50c09-en
- State Statistics Service of Ukraine. Available at: https://www.ukrstat.gov.ua/
- Batraga, A., Kite, M., Duboviks, J., Salkovska, J. (2020). Possible consequences of Brexit on European pharmaceutical market. New Challenges in Economic and Business Development – 2020: Economic Inequality and Well-Being, 54–64. Available at: https://dspace.lu.lv/dspace/bitstream/handle/7/54169/Batraga_A_Kite_M_Duboviks_J_Salkovska_J_NC_2020.pdf?sequence=1&isAllowed=y
- Valverde, J. L. (2016). The globalization of medicines as a challenge for governments. Pharmaceuticals Policy and Law, 18 (1–4), 19–29. doi: https://doi.org/10.3233/ppl-160429
- Kotvitska, A., Volkova, A., Korzh, I., Surikova, I. (2021). Comparative analysis of indicators that determine the effectiveness of the implementation of socio-economic determinants of health in Europe and Ukraine. ScienceRise: Pharmaceutical Science, 3 (31), 34–41. doi: https://doi.org/10.15587/2519-4852.2021.235787
- Bondarieva, I., Malyi, V., Posilkina, O., Mala, Z., Nessonova, M. (2021). Scientific and methodological approaches to modeling the optimal strategy for increasing the competitiveness of pharmacy chains of different sizes. ScienceRise: Pharmaceutical Science, 4 (32), 59–66. doi: https://doi.org/10.15587/2519-4852.2021.239389
- Rémuzat, C., Urbinati, D., Kornfeld, Å., Vataire, A.-L., Cetinsoy, L., Aballéa, S., Mzoughi, O., Toumi, M. (2014). Pharmaceutical expenditure forecast model to support health policy decision making. Journal of Market Access & Health Policy, 2 (1), 23740. doi: https://doi.org/10.3402/jmahp.v2.23740
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Oleh Samborskyi, Нanna Panfilova, Yuliia Baihush, Liusine Simonian, Iryna Bilyk, Tetiana Martyniuk, Halyna Tsikhon, Vitaly Chernukha

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






