SGLT-2 inhibitors as potential anticonvulsants: empagliflozin, but not dapagliflozin, renders a pronounced effect and potentiates the sodium valproate activity in pentylenetetrazole-induced seizures
DOI:
https://doi.org/10.15587/2519-4852.2022.266065Keywords:
anti-epileptic medicines, adjuvants, inhibitors of SGLT-2, chemo-induced seizures, miceAbstract
On the way to the search for effective adjuvant medicines for epilepsy treatment, antidiabetic medicines such as sodium-glucose cotransporter-2 inhibitors, which are expressed not only in the kidneys but also in the brain, attract attention. From previous studies, it is known that dapagliflozin improves electroencephalographic parameters in rats on the model of pentylenetetrazole-induced seizures. However, the anticonvulsant potential of other medicines from this group needs to be clarified.
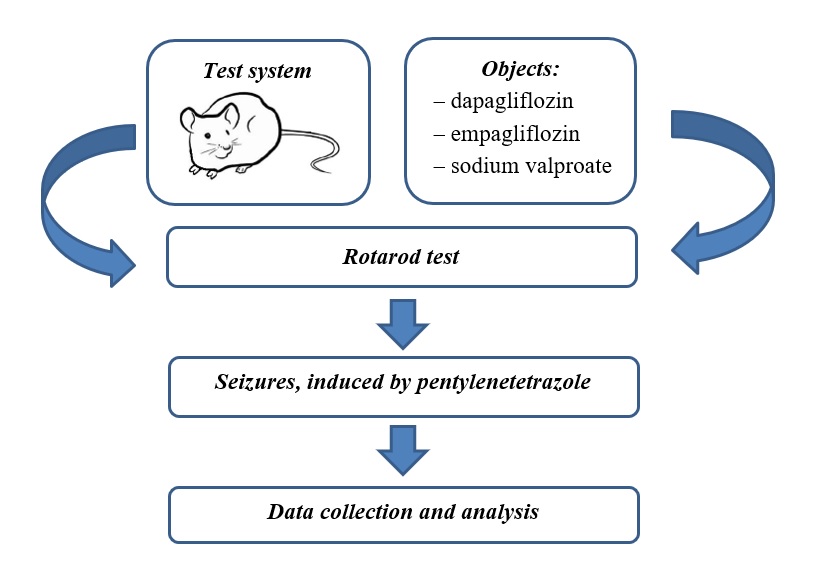
The aim of the study is to estimate the effect of empagliflozin, dapagliflozin per se and their combinations with sodium valproate on pentylenetetrazole-induced seizures, as well as on muscle tone and motor coordination in mice.
Material and methods. 42 random-bred male albino mice weighing 24-28 g were used in the experiments. Empagliflozin (20 mg/kg) and dapagliflozin (50 mg/kg) were administered intragastrically for 3 days. The classic anticonvulsant sodium valproate (150 mg/kg) per se, in combination with the medicines mentioned above, was administered in a similar regimen. On the second day, 30 minutes after administering all medicines, their effect on muscle tone and coordination of movements was determined in the rotarod test. On the third day, 30 minutes after the last administration of the medicines, their effect on pentylenetetrazole-induced (80 mg/kg subcutaneously) seizures was studied.
Results. For the first time, a pronounced anticonvulsant effect of empagliflozin was established both when used alone (a significant increase in latency of the convulsions and a decrease in lethality by 43 %) and especially in combination with sodium valproate (a significant increase in latency of the convulsions, a decrease in the number and severity of seizures and a decrease in lethality by 83 %), as well as the absence of a muscle relaxant effect in both cases. Dapagliflozin has neither its anticonvulsant properties nor its effect on the action of sodium valproate. However, this medicine caused muscle relaxation, especially when combined with sodium valproate.
Conclusions. The results suggest that empagliflozin, unlike dapagliflozin, has a high potential as an adjuvant medicine in treating epilepsy, as it enhances the efficacy of the classic anticonvulsant sodium valproate without muscle relaxant side effects
Supporting Agency
- No. 0120U102460 "Rationale for improving the treatment of multidrug-resistant epilepsy through the combined use of classic anticonvulsants with other medicines" (2020/2022), which is performed at the expense of the State Budget of Ukraine.
References
- Scheffer, I. E., Berkovic, S., Capovilla, G., Connolly, M. B., French, J., Guilhoto, L. et. al. (2017). ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia, 58 (4), 512–521. doi: https://doi.org/10.1111/epi.13709
- Abramovici, S., Bagić, A. (2016). Epidemiology of epilepsy. Handbook of clinical neurology, 138, 159–171. doi: https://doi.org/10.1016/B978-0-12-802973-2.00010-0
- Kalilani, L., Sun, X., Pelgrims, B., Noack-Rink, M., Villanueva, V. (2018). The epidemiology of drug-resistant epilepsy: A systematic review and meta-analysis. Epilepsia, 59 (12), 2179–2193. doi: https://doi.org/10.1111/epi.14596
- Copeland, L., Meek, A., Kerr, M., Robling, M., Hood, K., McNamara, R. (2017). Measurement of side effects of anti-epileptic drugs (AEDs) in adults with intellectual disability: A systematic review. Seizure, 51, 61–73. doi: https://doi.org/10.1016/j.seizure.2017.07.013
- Radu, B. M., Epureanu, F. B., Radu, M., Fabene, P. F., Bertini, G. (2017). Nonsteroidal anti-inflammatory drugs in clinical and experimental epilepsy. Epilepsy Research, 131, 15–27. doi: https://doi.org/10.1016/j.eplepsyres.2017.02.003
- Dhir, A. (2018). An update of cyclooxygenase (COX)-inhibitors in epilepsy disorders. Expert Opinion on Investigational Drugs, 28 (2), 191–205. doi: https://doi.org/10.1080/13543784.2019.1557147
- Zaccara, G., Lattanzi, S. (2019). Comorbidity between epilepsy and cardiac arrhythmias: Implication for treatment. Epilepsy & Behavior, 97, 304–312. doi: https://doi.org/10.1016/j.yebeh.2019.05.038
- Borowicz-Reutt, K. K. (2022). Effects of Antiarrhythmic Drugs on Antiepileptic Drug Action –A Critical Review of Experimental Findings. International Journal of Molecular Sciences, 23 (5), 2891. doi: https://doi.org/10.3390/ijms23052891
- Sawicka, K. M., Wawryniuk, A., Zwolak, A., Daniluk, J., Szpringer, M., Florek-Luszczki, M. et. al. (2017). Influence of Ivabradine on the Anticonvulsant Action of Four Classical Antiepileptic Drugs Against Maximal Electroshock-Induced Seizures in Mice. Neurochemical Research, 42 (4), 1038–1043. doi: https://doi.org/10.1007/s11064-016-2136-1
- Togha, M., Akhondzadeh, S., Motamedi, M., Ahmadi, B., Razeghi, S. (2007). Allopurinol as Adjunctive Therapy in Intractable Epilepsy: A Double-blind and Placebo-controlled Trial. Archives of Medical Research, 38 (3), 313–316. doi: https://doi.org/10.1016/j.arcmed.2006.10.010
- Quintana-Pájaro, L. D. J., Ramos-Villegas, Y., Cortecero-Sabalza, E., Joaquim, A. F., Agrawal, A., Narvaez-Rojas, A. R., Moscote-Salazar, L. R. (2018). The Effect of Statins in Epilepsy: A Systematic Review. Journal of Neurosciences in Rural Practice, 9 (4), 478–486. doi: https://doi.org/10.4103/jnrp.jnrp_110_18
- Scicchitano, F., Constanti, A., Citraro, R., Sarro, G., Russo, E. (2015). Statins and epilepsy: preclinical studies, clinical trials and statin-anticonvulsant drug interactions. Current Drug Targets, 16 (7), 747–756. doi: https://doi.org/10.2174/1389450116666150330114850
- Tawfik, K. M., Moustafa, Y. M., El-Azab, M. F. (2018). Neuroprotective mechanisms of sildenafil and selenium in PTZ-kindling model: Implications in epilepsy. European Journal of Pharmacology, 833, 131–144. doi: https://doi.org/10.1016/j.ejphar.2018.05.035
- Aygun, H., Bilginoglu, A. (2019). Effect of tadalafil and nitric oxide agonist sodium nitroprusside on penicillin-induced epileptiform activity. Neurological Research, 42 (1), 39–46. doi: https://doi.org/10.1080/01616412.2019.1703166
- Tsyvunin, V., Shtrygol’, S., Shtrygol’, D. (2020). Digoxin enhances the effect of antiepileptic drugs with different mechanism of action in the pentylenetetrazole-induced seizures in mice. Epilepsy Research, 167, 106465. doi: https://doi.org/10.1016/j.eplepsyres.2020.106465
- Tsyvunin, V., Shtrygol’, S., Shtrygol', D., Mishchenko, M., Kapelka, I. Taran, A. (2021). Digoxin potentiates the anticonvulsant effect of carbamazepine and lamotrigine against experimental seizures in mice. Thai Journal of Pharmaceutical Sciences, 45 (3), 165–171.
- Tsyvunin, V., Shtrygol’, S., Havrylov, I., Shtrygol’, D. (2021). Low-dose digoxin enhances the anticonvulsive potential of carbamazepine and lamotrigine in chemo-induced seizures with different neurochemical mechanisms. ScienceRise: Pharmaceutical Science, 6 (34), 58–65. doi: https://doi.org/10.15587/2519-4852.2021.249375
- Tsyvunin, V., Shtrygol, S., Mishchenko, M., Shtrygol, D. (2022). Digoxin at sub-cardiotonic dose modulates the anticonvulsive potential of valproate, levetiracetam and topiramate in experimental primary generalized seizures. Česká a Slovenská Farmacie, 71 (2), 76–86. doi: https://doi.org/10.5817/csf2022-2-76
- Tentolouris, A., Vlachakis, P., Tzeravini, E., Eleftheriadou, I., Tentolouris, N. (2019). SGLT2 Inhibitors: A Review of Their Antidiabetic and Cardioprotective Effects. International Journal of Environmental Research and Public Health, 16 (16), 2965. doi: https://doi.org/10.3390/ijerph16162965
- Zinman, B., Wanner, C., Lachin, J. M., Fitchett, D., Bluhmki, E., Hantel, S. et. al. (2015). Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. New England Journal of Medicine, 373 (22), 2117–2128. doi: https://doi.org/10.1056/nejmoa1504720
- Neal, B., Perkovic, V., Mahaffey, K. W., de Zeeuw, D., Fulcher, G., Erondu, N. et. al. (2017). Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. New England Journal of Medicine, 377 (7), 644–657. doi: https://doi.org/10.1056/nejmoa1611925
- Yu, A. S., Hirayama, B. A., Timbol, G., Liu, J., Diez-Sampedro, A., Kepe, V. et. al. (2013). Regional distribution of SGLT activity in rat brain in vivo. American Journal of Physiology-Cell Physiology, 304 (3), C240–C247. doi: https://doi.org/10.1152/ajpcell.00317.2012
- Wright, E. M., Loo, D. D. F., Hirayama, B. A. (2011). Biology of Human Sodium Glucose Transporters. Physiological Reviews, 91 (2), 733–794. doi: https://doi.org/10.1152/physrev.00055.2009
- Melo, I. S., Santos, Y. M. O., Costa, M. A., Pacheco, A. L. D., Silva, N. K. G. T., Cardoso-Sousa, L. et. al. (2016). Inhibition of sodium glucose cotransporters following status epilepticus induced by intrahippocampal pilocarpine affects neurodegeneration process in hippocampus. Epilepsy & Behavior, 61, 258–268. doi: https://doi.org/10.1016/j.yebeh.2016.05.026
- Erdogan, M. A., Yusuf, D., Christy, J., Solmaz, V., Erdogan, A., Taskiran, E., Erbas, O. (2018). Highly selective SGLT2 inhibitor dapagliflozin reduces seizure activity in pentylenetetrazol-induced murine model of epilepsy. BMC Neurology, 18 (1). doi: https://doi.org/10.1186/s12883-018-1086-4
- Blunck, J. R., Newman, J. W., Fields, R. K., Croom, J. E. (2018). Therapeutic augmentation of ketogenic diet with a sodium-glucose cotransporter 2 inhibitor in a super-refractory status epilepticus patient. Epilepsy & Behavior Case Reports, 10, 61–64. doi: https://doi.org/10.1016/j.ebcr.2018.05.002
- Bodenant, M., Moreau, C., Sejourné, C., Auvin, S., Delval, A., Cuisset, J. M. et. al. (2008). Interest of the ketogenic diet in a refractory status epilepticus in adults. Revue neurologique, 164 (2), 194–199. doi: https://doi.org/10.1016/j.neurol.2007.08.009
- Ogawa, W., Sakaguchi, K. (2015). Euglycemic diabetic ketoacidosis induced by SGLT2 inhibitors: possible mechanism and contributing factors. Journal of Diabetes Investigation, 7 (2), 135–138. doi: https://doi.org/10.1111/jdi.12401
- Lidster, K., Jefferys, J. G., Blümcke, I., Crunelli, V., Flecknell, P., Frenguelli, B. G. et. al. (2016). Opportunities for improving animal welfare in rodent models of epilepsy and seizures. Journal of Neuroscience Methods, 260, 2–25. doi: https://doi.org/10.1016/j.jneumeth.2015.09.007
- Deacon, R. M. J. (2013). Measuring Motor Coordination in Mice. Journal of Visualized Experiments, 75. doi: https://doi.org/10.3791/2609
- Hock, F. J. (Ed.) (2016). Drug Discovery and Evaluation: Pharmacological Assays. Springer International Publishing, 4314. doi: http://doi.org/10.1007/978-3-319-05392-9
- Glantc, S. (1999). Mediko-biologicheskaia statistika. Moscow: Praktika, 459.
- Nambu, H., Takada, S., Fukushima, A., Matsumoto, J., Kakutani, N., Maekawa, S. et. al. (2020). Empagliflozin restores lowered exercise endurance capacity via the activation of skeletal muscle fatty acid oxidation in a murine model of heart failure. European Journal of Pharmacology, 866, 172810. doi: https://doi.org/10.1016/j.ejphar.2019.172810
- Arab, H. H., Safar, M. M., Shahin, N. N. (2021). Targeting ROS-Dependent AKT/GSK-3β/NF-κB and DJ-1/Nrf2 Pathways by Dapagliflozin Attenuates Neuronal Injury and Motor Dysfunction in Rotenone-Induced Parkinson’s Disease Rat Model. ACS Chemical Neuroscience, 12 (4), 689–703. doi: https://doi.org/10.1021/acschemneuro.0c00722
- Yun, C., Xuefeng, W. (2013). Association Between Seizures and Diabetes Mellitus: A Comprehensive Review of Literature. Current Diabetes Reviews, 9 (4), 350–354. doi: https://doi.org/10.2174/15733998113099990060
- Meidenbauer, J. J., Roberts, M. F. (2014). Reduced glucose utilization underlies seizure protection with dietary therapy in epileptic EL mice. Epilepsy & Behavior, 39, 48–54. doi: https://doi.org/10.1016/j.yebeh.2014.08.007
- Rovet, J. F., Ehrlich, R. M. (1999). The effect of hypoglycemic seizures on cognitive function in children with diabetes: A 7-year prospective study. The Journal of Pediatrics, 134 (4), 503–506. doi: https://doi.org/10.1016/s0022-3476(99)70211-8
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Vadim Tsyvunin, Sergiy Shtrygol’, Ihnat Havrylov, Diana Shtrygol’, Artur Reus

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






