Development of the spectrophotometric method for the determination of metoprolol tartrate in tablets by using bromocresol green
DOI:
https://doi.org/10.15587/2519-4852.2022.266068Keywords:
bromocresol green, metoprolol, spectrophotometry, validation, quantitative determination, tabletsAbstract
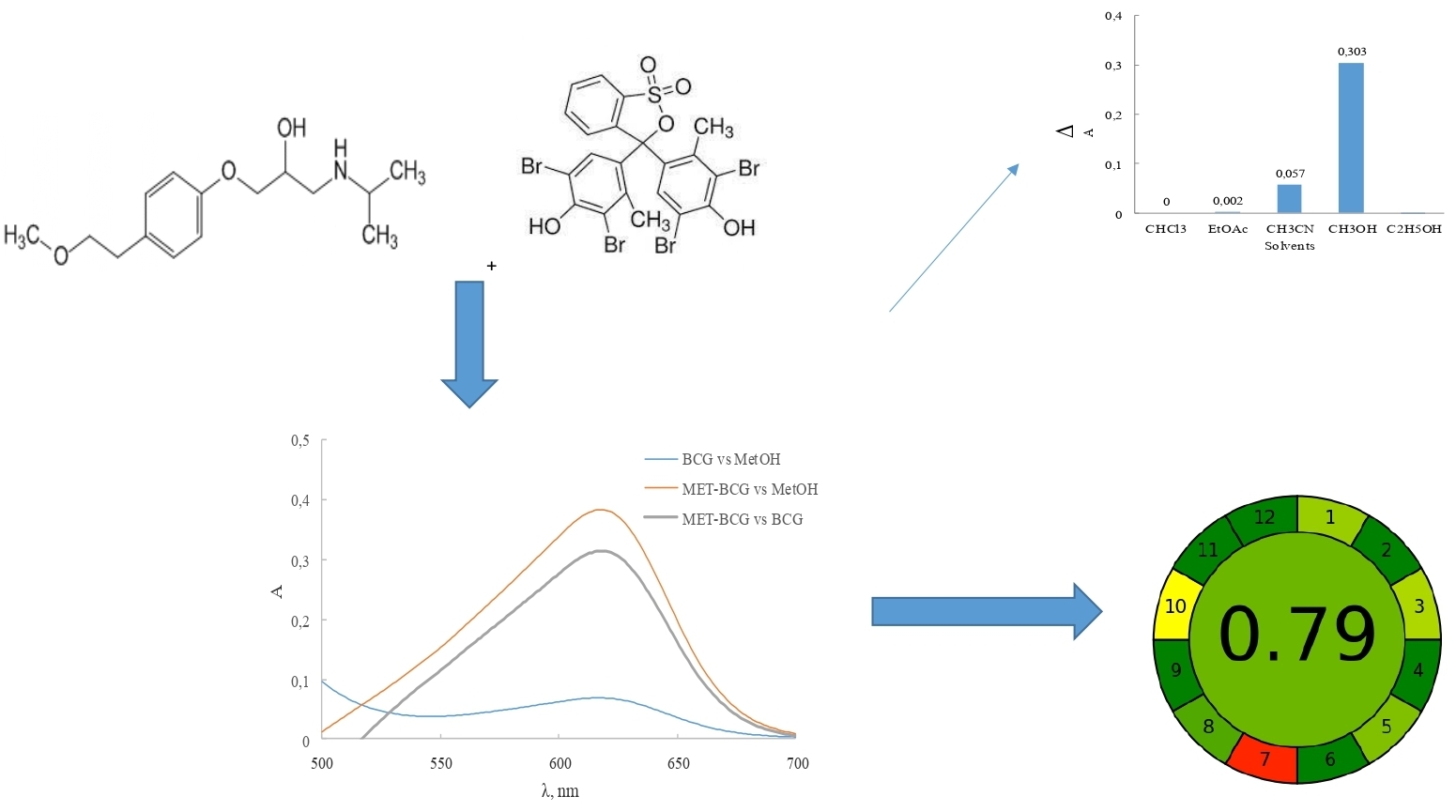
The aim of the work was to develop and validate a spectrophotometric method for determining metoprolol tartrate in tablets based on the reaction with bromocresol green (BCG).
Material and methods. Analytical equipment: two-beam UV-visible spectrophotometer Shimadzu model -UV 1800 (Japan), software UV-Probe 2.62, laboratory electronic balance RAD WAG AS 200/C, pH-meter И-160МИ. The following APIs, dosage forms, reagents and solvents were used in the work: pharmacopoeial standard sample (CRS) of metoprolol tartrate (Sigma-Aldrich, (≥ 98 %, HPLC)), BCG (Sigma-Aldrich, (≥ 98 %, HPLC)), "Metoprolol" tablets 50 mg (Kyivmedpreparat, series 0035415), "Metoprolol" 100 mg (Farmak, series 30421), methanol (Honeywell, (≥ 99.9 %, GC)), ethanol (Honeywell, (≥ 99.9 %, GC)), chloroform (Honeywell, (≥ 99.9 %, GC)), acetonitrile (Honeywell, (≥ 99.9 %, GC)), and ethyl acetate (Honeywell, (≥ 99.7 %, GC)).
Results and discussion. A spectrophotometric method was developed for determining metoprolol tartrate by reaction with BCG in a methanol solution using the absorption maximum at a wavelength of 624 nm. Stoichiometric ratios of reactive components were established, which were 1:1. The developed method for the quantitative determination of metoprolol tartrate was validated following the requirements of the SPhU. The analytical method was linear in the concentration range of 5.47-38.30 μg/mL. The limit of detection and quantification were 0.41 μg/mL and 1.24 μg/mL, respectively. According to the «greenness» pictogram of the analytical method using the AGREE method, the score was 0.79, which indicates that the proposed spectrophotometric method for the determination of metoprolol was developed in compliance with the principles of «green» chemistry.
Conclusions. A spectrophotometric method for determining metoprolol tartrate in tablets based on the reaction with BCG in compliance with the principles of «green» chemistry has been developed and validated. Furthermore, the developed method for the quantitative determination of metoprolol tartrate was validated following the requirements of the SPhU. In summary, the developed method has a low negative impact on the environment and can be applied for routine pharmaceutical analysis
Supporting Agency
- Ministry of Health of Ukraine under project number 0120U104201
References
- Tucker, W. D., Sankar, P., Kariyanna, P. Th. (2022). Selective Beta-1-Blockers. StatPearls. Available at: https://www.ncbi.nlm.nih.gov/books/NBK499982/#:~:text=The%20cardio%2Dselective%20beta%2D1,acebutolol%2C%20metoprolol%2C%20and%20nebivolol
- Metoprolol tartrate. Available at: https://go.drugbank.com/salts/DBSALT000862
- European Pharmacopoeia (2020). Available at: https:.www.edqm.eu/en/european-pharmacopoeia-ph-eur-10th-edition
- Cesme, M., Tarinc, D., Golcu, A. (2011). Spectrophotometric Determination of Metoprolol Tartrate in Pharmaceutical Dosage Forms on Complex Formation with Cu(II). Pharmaceuticals, 4 (7), 964–975. doi: https://doi.org/10.3390/ph4070964
- Nabil, A. F., Eman, M. S. (2015). Spectrophotometric determination of metoprolol in pharmaceutical formulation by charge transfer complexation. International Journal of Chemical Studies, 3, 24–29. Available at: https://www.academia.edu/16235931/Spectrophotometric_determination_of_metoprolol_in_pharmaceutical_formulation_by_charge_transfer_complexation
- Donchenko, A., Vasyuk, S. (2018). Spectrophotometric determination of metoprolol tartrate in pure and dosage forms. Ankara Üniversitesi Eczacılık Fakültesi Dergisi, 42 (1), 33–42. doi: https://doi.org/10.1501/eczfak_0000000600
- Jadhav, A. S., Tarkase, K. N., Deshpande, A. P. (2012). Quantitative determination of metoprolol succinate in bulk and tablet dosage form through comparative study of UV and derivative Spectroscopy. Der Pharmacia Lettre, 4, 763–767. Available at: https://www.scholarsresearchlibrary.com/articles/quantitative-determination-of-metoprolol-succinate-in-bulk-and-tablet-dosage-form-through-comparative-study-of-uv-and-de.pdf
- Rahman, N., Rahman, H., Azmi, S. N. H. (2005). Validated Kinetic Spectrophotometric Method for the Determination of Metoprolol Tartrate in Pharmaceutical Formulations. Chemical and Pharmaceutical Bulletin, 53 (8), 942–948. doi: https://doi.org/10.1248/cpb.53.942
- Hussain, S., Munjewar, R. R., Farooqui, M. (2012). Development and validation of a simultaneous HPLC method for quantification of amlodipine besylate and metoprolol tartrate in tablets. Journal of PharmaSciTech, 1, 1–5. Available at: http://www.pharmascitech.in/admin/php/uploads/32_pdf.pdf
- Brijesh, S., Patel, D., Ghosh, S. (2009). Development of Reverse-Phase HPLC Method for Simultaneous Analysis of Metoprolol Succinate and Hydrochlorothiazide in a Tablet Formulation. Tropical Journal of Pharmaceutical Research, 8 (6), 539–543. doi: https://doi.org/10.4314/tjpr.v8i6.49401
- Prasad, P. H., Patel, P. M., Vijaysree, D., Reddy, Y. S., Ranjith, K. B. (2013). Simultaneous estimation of metoprolol tartrate and chlorthalidone by using RP-HPLC and method development as per ICH guidelines. Der Pharma Chemica, 5, 139–143. Available at: https://www.derpharmachemica.com/pharma-chemica/simultaneous-estimation-of-metoprolol-tartrate-and-chlorthalidone-by-using-rphplc-and-method-development-as-per-ich-guid.pdf
- Mahaparale, S. P., Gonjari, I. D., Jayaveera, K. N. (2013). Stability indicating hplc method for simultaneous estimation of metoprolol succinate and telmisartan. Journal of Liquid Chromatography & Related Technologies, 36 (18), 2601–2611. doi: https://doi.org/10.1080/10826076.2012.723095
- Braza, A. J., Modamio, P., Lastra, C. F., Mariño, E. L. (2002). Development, validation and analytical error function of two chromatographic methods with fluorimetric detection for the determination of bisoprolol and metoprolol in human plasma. Biomedical Chromatography, 16 (8), 517–522. doi: https://doi.org/10.1002/bmc.195
- Albers, S., Elshoff, J.-P., Völker, C., Richter, A., Läer, S. (2004). HPLC quantification of metoprolol with solid-phase extraction for the drug monitoring of pediatric patients. Biomedical Chromatography, 19 (3), 202–207. doi: https://doi.org/10.1002/bmc.436
- Chiu, F. C. K., Damani, L. A., Li, R. C., Tomlinson, B. (1997). Efficient high-performance liquid chromatographic assay for the simultaneous determination of metoprolol and two main metabolites in human urine by solid-phase extraction and fluorescence detection. Journal of Chromatography B: Biomedical Sciences and Applications, 696 (1), 69–74. doi: https://doi.org/10.1016/s0378-4347(97)00059-5
- Johnson, R. D., Lewis, R. J. (2006). Quantitation of atenolol, metoprolol, and propranolol in postmortem human fluid and tissue specimens via LC/APCI-MS. Forensic Science International, 156 (2-3), 106–117. doi: https://doi.org/10.1016/j.forsciint.2005.01.001
- Jensen, B. P., Sharp, C. F., Gardiner, S. J., Begg, E. J. (2008). Development and validation of a stereoselective liquid chromatography–tandem mass spectrometry assay for quantification of S- and R-metoprolol in human plasma. Journal of Chromatography B, 865 (1-2), 48–54. doi: https://doi.org/10.1016/j.jchromb.2008.02.006
- Gowda, K. V., Mandal, U., Senthamil Selvan, P., Sam Solomon, W. D., Ghosh, A., Sarkar, A. K., Agarwal, S. et. al. (2007). Liquid chromatography tandem mass spectrometry method for simultaneous determination of metoprolol tartrate and ramipril in human plasma. Journal of Chromatography B, 858 (1-2), 13–21. doi: https://doi.org/10.1016/j.jchromb.2007.07.047
- Issa, Y. M., Abdel-Gawad, F. M., Abou Table, M. A., Hussein, H. M. (1997). Spectrophotometric Determination of Ofloxacin and Lomefloxacin Hydrochloride with Some Sulphonphthalein Dyes. Analytical Letters, 30 (11), 2071–2084. doi: https://doi.org/10.1080/00032719708001722
- Prashanth, K., Basavaiah, K., Raghu, M. (2012). Rapid and sensitive spectrophotometric measurement of non-specific beta blocker propranolol hydrochloride using three sulphonphthalein dyes in pure form, pharmaceuticals and human urine. Chemical Sciences Journal, 2012, 2 14. Available at: https://www.hilarispublisher.com/open-access/rapid-and-sensitive-spectrophotometric-measurement-of-non-specific-beta-blocker-propranolol-hydrochloride-.2150-3494.1000056.pdf
- El-Yazbi, F. A., Gazy, A. A., Mahgoub, H., El-Sayed, M. A., Youssef, R. M. (2003). Spectrophotometric and titrimetric determination of nizatidine in capsules. Journal of Pharmaceutical and Biomedical Analysis, 31 (5), 1027–1034. doi: https://doi.org/10.1016/s0731-7085(02)00699-4
- Abdine, H., Belal, F., Zoman, N. (2002). Simple spectrophotometric determination of cinnarizine in its dosage forms. Il Farmaco, 57 (4), 267–271. doi: https://doi.org/10.1016/s0014-827x(02)01204-1
- Derayea, S. M. S. (2014). An application of eosin Y for the selective spectrophotometric and spectrofluorimetric determination of mebeverine hydrochloride. Anal. Methods, 6 (7), 2270–2275. doi: https://doi.org/10.1039/c3ay41371c
- Derzhavna Farmakopeia Ukraini. Vol. 1 (2015). Kharkіv: Derzhavne pіdpriemstvo «Naukovo-ekspertnii farmakopeinii tcentr», 1128.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Mariana Horyn, Tetyana Kucher, Liubomyr Kryskiw, Olha Poliak, Nadiya Zarivna, Kateryna Peleshok, Liliya Logoyda

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






