Development of the spectrophotometric method for the determination of rosuvastatin in tablets by using bromophenol blue
DOI:
https://doi.org/10.15587/2519-4852.2023.277461Keywords:
bromophenol blue, rosuvastatin, spectrophotometry, validation, quantitative determination, tabletsAbstract
The aim of the work was to develop a spectrophotometric method for the determination of rosuvastatin in tablets based on the reaction with BPB in compliance with the principles of «green» chemistry.
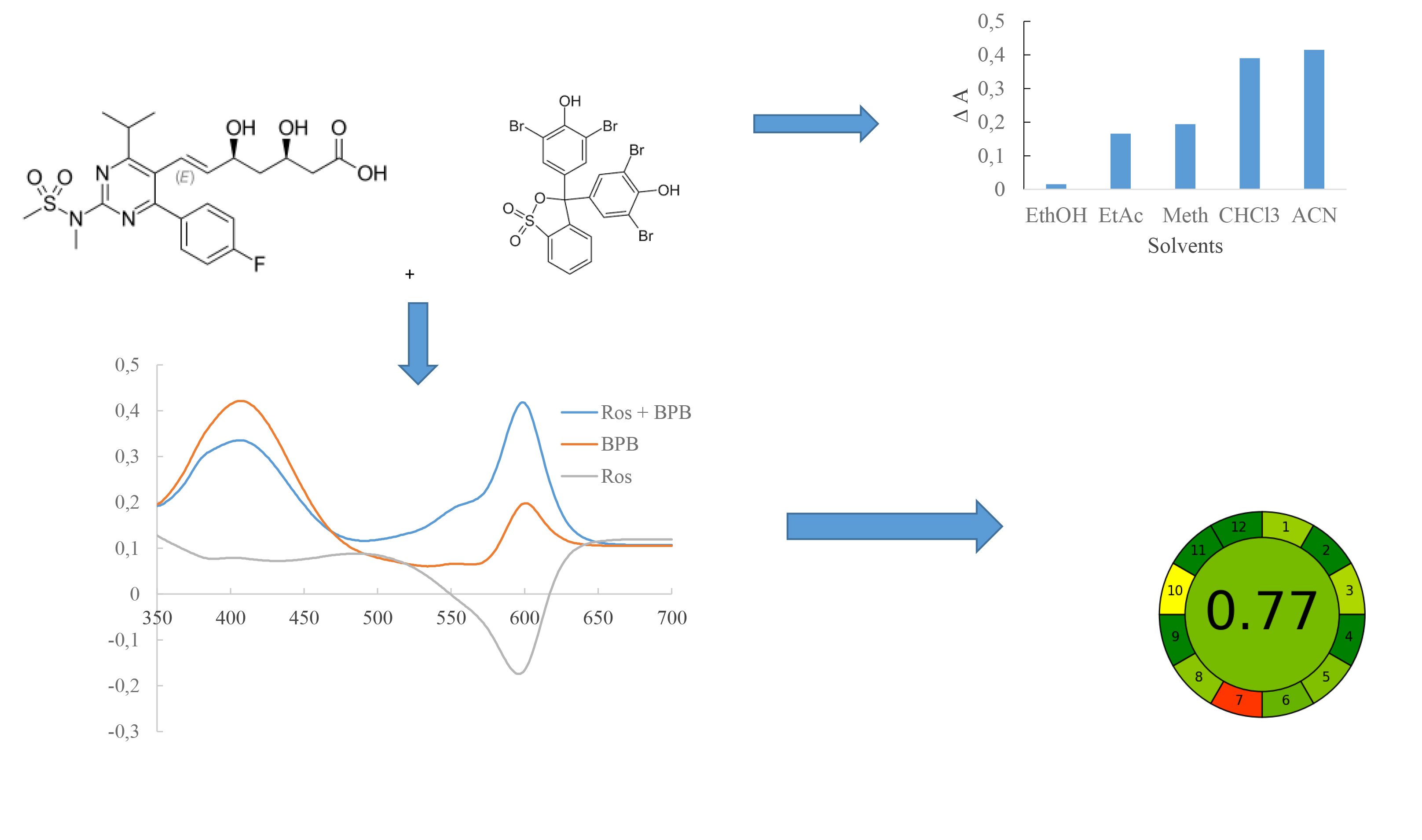
Material and methods. Analytical equipment: two-beam UV-visible spectrophotometer Shimadzu model -UV 1800 (Japan), software UV-Probe 2.62, electronic laboratory balance RAD WAG AS 200/C. The following APIs, dosage forms, reagents and solvents were used in work: pharmacopoeial standard sample (CRS) of rosuvastatin calcium (Sigma-Aldrich, (≥ 98 %, HPLC)), BCG (Sigma-Aldrich, (≥ 98 %, HPLC)), "Rosuvastatin" tablets 10 mg, 15 mg, 20 mg, methanol (Honeywell, (≥ 99.9 %, GC)), ethanol (Honeywell, (≥ 99.9 %, GC)), chloroform (Honeywell, (≥ 99.9 %, GC)), acetonitrile (Honeywell, (≥ 99.9 %, GC)), and ethyl acetate (Honeywell, (≥ 99.7 %, GC)).
Results and discussion. A spectrophotometric method for determining rosuvastatin by reaction with BPB in an acetonitrile solution using the absorption maximum at a wavelength of 595 nm has been developed. Stoichiometric ratios of reactive components were established, which were 1:1. The developed method for the quantitative determination of rosuvastatin was validated following the requirements of the SPhU. The analytical method was linear in the 7.99-23.97 μmol/L concentration range. The LOD and LOQ values were calculated to be 0.77 µmol/L and 2.36 µmol/L. According to the «greenness» pictogram of the analytical method using the AGREE method, the score was 0.77, indicating that the proposed spectrophotometric method for determining rosuvastatin was developed in compliance with the principles of «green» chemistry.
Conclusions. An eco-friendly spectrophotometric method has been developed to quantitatively determine rosuvastatin in tablets based on the reaction with BPB. The appropriate sulfophthalein dye (BPB) and its concentration (4.00 x 10-4), the optimal eco-friendly solvent (acetonitrile), and the appropriate wavelength (595 nm) were chosen, and the sensitivity of the reaction was calculated. The analytical method was validated, and its possibility for use in the pharmaceutical analysis was shown
References
- Barna, O. M. (2013). Efficient and effective cardiovascular prevention: the role of rosuvastatin. Ukrainskyi medychnyi chasopys, 5 (97), 93–98.
- Vapor Pressure. Rosuvastatin. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/Rosuvastatin#section=Vapor-Pressure
- Buckett, L., Ballard, P., Davidson, R., Dunkley, C., Martin, L., Stafford, J., McTaggart, F. (2000). Selectivity of ZD4522 for inhibition of cholesterol synthesis in hepatic versus non-hepatic cells. Atherosclerosis, 151 (1), 41. doi: https://doi.org/10.1016/s0021-9150(00)80185-9
- Smith, G., Davidson, R., Bloor, S., Burns, K., Calnan, C., McAulay, P., Torr, N., Ward, W., McTaggart, F. (2000). Pharmacological properties of ZD4522 – A new HMG-CoA reductase inhibitor. Atherosclerosis, 151 (1), 39. doi: https://doi.org/10.1016/s0021-9150(00)80176-8
- European Pharmacopoeia (2022). Available at: https://www.edqm.eu/en/web/edqm/european-pharmacopoeia-ph.-eur.-11th-edition
- Ângelo, M. L., Moreira, F. de L., Morais Ruela, A. L., Santos, A. L. A., Salgado, H. R. N., de Araújo, M. B. (2018). Analytical Methods for the Determination of Rosuvastatin in Pharmaceutical Formulations and Biological Fluids: A Critical Review. Critical Reviews in Analytical Chemistry, 48 (4), 317–329. doi: https://doi.org/10.1080/10408347.2018.1439364
- Uyar, B., Celebier, M., Altinoz, S. (2007). Spectrophotometric determination of rosuvastatin calcium in tablets. Die Pharmazie-An International Journal of Pharmaceutical Sciences, 62 (6), 411–413.
- Gupta, A., Mishra, P., Shah, K. (2009). Simple UV Spectrophotometric Determination of Rosuvastatin Calcium in Pure Form and in Pharmaceutical Formulations. E-Journal of Chemistry, 6 (1), 89–92. doi: https://doi.org/10.1155/2009/956712
- Krishna, M. V., Sankar, D. G. (2007). Extractive Spectrophotometric Methods for the Determination of Rosuvastatin Calcium in Pure Form and in Pharmaceutical Formulations by Using Safranin O and Methylene blue. E-Journal of Chemistry, 4 (1), 46–49. doi: https://doi.org/10.1155/2007/454853
- Ramadan, A. A., Mandil, H. A. S. N. A., Alshelhawi, N. O. O. R. (2014). Spectrophotometric determination of rosuvastatin calcium in pure form and pharmaceutical formulations by the oxidation using iodine and formation triiodide complex in acetonitrile. International Journal of Pharmacy and Pharmaceutical Sciences, 6 (5), 579–585.
- Lima, M. F., Cassella, R. J., Pacheco, W. F. (2017). Spectrophotometric determination of rosuvastatin in pharmaceutical formulations using quinalizarin. Brazilian Journal of Pharmaceutical Sciences, 53 (3). doi: https://doi.org/10.1590/s2175-97902017000300075
- Braga, V. S. M., Mancilha, T. P., Cassella, R. J., Pacheco, W. F. (2012). Determination of Rosuvastatin in Urine by Spectrofluorimetry After Liquid–Liquid Extraction and Derivatization in Acidic Medium. Journal of Fluorescence, 23 (1), 49–55. doi: https://doi.org/10.1007/s10895-012-1115-4
- Ramadan, A. A., Mandil, H. A. S. N. A., Alsayed-Ali, R. A. F. I. F. (2015). Spectrophotometric determination of rosuvastatin in pure form and pharmaceutical formulations through ion-pair complex formation using bromocresol green. International Journal of Pharmacy and Pharmaceutical Sciences, 7 (11), 191–198.
- Prajapati, P. B., Bodiwala, K. B., Marolia, B. P., Rathod, I. S., Shah, S. A. (2010). Development and validation of extractive spectrophotometric method for determination of rosuvastatin calcium in pharmaceutical dosage forms. Journal of Pharmacy Research, 3 (8), 2036–2038.
- Ângelo, M. L. (2016). Análise químico-farmacêutica de rosuvastatina cálcica comprimido e cápsula. Brazil.
- Sevda, R. R., Ravetkar, A. S., Shirote, P. J. (2011). UV Spectrophotometric estimation of rosuvastatin calcium and fenofibrate in bulk drug and dosage form using simultaneous equation method. International Journal of ChemTech Research, 3 (2), 629–635.
- El-Bagary, R. I., ElKady, E. F., Kadry, A. M. (2012). Spectrofluorometric Determination of Certain Antihyperlipidemic Agents in Bulk and Pharmaceutical Preparations. Spectroscopy: An International Journal, 27, 83–92. doi: https://doi.org/10.1155/2012/913913
- Patel, B., Jadav, A., Solanki, H., Parmar, S., Parmar, V., Captain, A. (2013). Development and validation of derivative spectroscopic method for the simultaneous estimation of rosuvastatin calcium and fenofibrate in tablet. International Journal of Pharma Research & Review, 2 (7), 1–6.
- Ambole, S. R., Shirote, P. J., Kondawar, M. S. (2012). Simultaneous Estimation for Rosuvastatin calcium and Aspirin from Capsule Dosage Forms by First Order. Derivative Spectroscopic Method. International Journal of ChemTech Research, 4 (3), 966–970.
- Karunakaran, A., Subhash, V., Chinthala, R., Muthuvijayan, J. (1970). Simultaneous Estimation of Rosuvastatin Calcium and Fenofibrate in Bulk and in Tablet Dosage Form by UV-Spectrophotometry and RP-HPLC. Stamford Journal of Pharmaceutical Sciences, 4 (1), 58–63. doi: https://doi.org/10.3329/sjps.v4i1.8868
- Sharma, S., Bhandari, P. (2005). Simultaneous Estimation of Rosuvastatin Calcium and Fenofibrate in Bulk and in Tablet Dosage Form by UV-Spectrophotometry and RP-HPLC. Journal of Pharmacy Research, 5, 2311–2314.
- Afroz, A., Haque, T., Talukder, M. U., Islam, S. M. (2011). Spectrophotometric estimation of rosuvastatin calcium and glimepiride in tablet dosage form. Asian Journal of pharmaceutical analysis, 1 (4), 74–78.
- Parmar, V., Solanki, H., Prajapati, L. (2013). Derivative spectrophotometric determination of rosuvastatin calcium and fenofibrate in tablet dosage form. Inventi Rapid: Pharm Analysis & Quality Assurance, 2, 1–5.
- Dudhipala, N., Veerabrahma, K. (2017). Improved anti-hyperlipidemic activity of Rosuvastatin Calcium via lipid nanoparticles: Pharmacokinetic and pharmacodynamic evaluation. European Journal of Pharmaceutics and Biopharmaceutics, 110, 47–57. doi: https://doi.org/10.1016/j.ejpb.2016.10.022
- Nazir, S., Iqbal, Z., Nasir, F. (2015). Impact of Menopause on Pharmacokinetics of Rosuvastatin Compared with Premenopausal Women. European Journal of Drug Metabolism and Pharmacokinetics, 41 (5), 505–509. doi: https://doi.org/10.1007/s13318-015-0285-2
- Beludari, M. I., Prakash, K. V., Mohan, G. K. (2013). RP-HPLC method for simultaneous estimation of Rosuvastatin and Ezetimibe from their combination tablet dosage form. International Journal of Chemical and Analytical Science, 4 (4), 205–209. doi: https://doi.org/10.1016/j.ijcas.2013.04.006
- Balakumar, K., Raghavan, C. V., selvan, N. T., prasad, R. H., Abdu, S. (2013). Self nanoemulsifying drug delivery system (SNEDDS) of Rosuvastatin calcium: Design, formulation, bioavailability and pharmacokinetic evaluation. Colloids and Surfaces B: Biointerfaces, 112, 337–343. doi: https://doi.org/10.1016/j.colsurfb.2013.08.025
- Kumar, T. R., Shitut, N. R., Kumar, P. K., Vinu, M. C. A., Kumar, V. V. P., Mullangi, R., Srinivas, N. R. (2006). Determination of rosuvastatin in rat plasma by HPLC: validation and its application to pharmacokinetic studies. Biomedical Chromatography, 20(9), 881–887. doi: https://doi.org/10.1002/bmc.611
- Caglar, S., Toker, S. (2012). Determination of Rosuvastatin at Picogram Level in Serum by Fluorimetric Derivatization with 9-Anthryldiazomethane using HPLC. Journal of Chromatographic Science, 51 (1), 53–58. doi: https://doi.org/10.1093/chromsci/bms105
- Eswarudu, M. M., Mounica, P., Venkatesh, D., Nagalakshmi, B. (2012). Method Development and Validation for Simultaneous Estimation of Rosuvastatin Calcium and Ezetimibe in Pharmaceutical Dosage Form by RP-HPLC. International Research Journal of Pharmaceutical and Applied Sciences, 2, 24–36.
- Trivedi, H. K., Patel, M. C. (2012). Development and Validation of a Stability-Indicating RP-UPLC Method for Determination of Rosuvastatin and Related Substances in Pharmaceutical Dosage Form. Scientia Pharmaceutica, 80 (2), 393–406. doi: https://doi.org/10.3797/scipharm.1201-09
- Rao, A. L., Suneetha, D. (2010). Development and validation of RP-HPLC method for the estimation of rosuvastatin in bulk and pharmaceutical dosage form. International Journal of Chemical Science, 8 (2), 1308–1314.
- Haq, N., Shakeel, F., Alanazi, F., Alshora, D. H., Ibrahim, M. A. (2018). Development and validation of a green RP-HPLC method for the analysis of rosuvastatin: a step towards making liquid chromatography environmentally benign. Green Processing and Synthesis, 7 (2), 160–169. doi: https://doi.org/10.1515/gps-2017-0023
- Mostafa, N. M., Badawey, A. M., Lamie, N. T., Abd El-Aleem, A. E. A. B. (2014). Selective chromatographic methods for the determination of Rosuvastatin calcium in the presence of its acid degradation products. Journal of Liquid Chromatography & Related Technologies, 37 (15), 2182–2196. doi: https://doi.org/10.1080/10826076.2013.828305
- Kaila, H., Ambasana, M., Thakkar, R., Saravaia, H., Shah, A. (2010). A new improved RP-HPLC method for assay of rosuvastatin calcium in tablets. Indian Journal of Pharmaceutical Sciences, 72 (5), 592–598. doi: https://doi.org/10.4103/0250-474x.78526
- Hassouna, M. K. M., Abdel-Mageed, A. I., Salem, H. O. (2017). Indirect Oxygen Flask-Atomic Absorption Spectrometric Determination of Rosuvastatin Calcium. Biomedical Journal of Scientific & Technical Research, 1, 1–6. doi: https://doi.org/10.26717/bjstr.2017.01.000164
- Sree Janardhanan, V., Manavalan, R., Valliappan, K. (2016). Chemometric technique for the optimization of chromatographic system: Simultaneous HPLC determination of Rosuvastatin, Telmisartan, Ezetimibe and Atorvastatin used in combined cardiovascular therapy. Arabian Journal of Chemistry, 9, S1378–S1387. doi: https://doi.org/10.1016/j.arabjc.2012.03.001
- Kishore, C. R. P., Mohan, G. V. K. (2017). Structural identification and estimation of Rosuvastatin calcium related impurities in Rosuvastatin calcium tablet dosage form. Analytical Chemistry Research, 12, 17–27. doi: https://doi.org/10.1016/j.ancr.2016.11.002
- Shah, Y., Iqbal, Z., Ahmad, L., Khan, A., Khan, M. I., Nazir, S., Nasir, F. (2011). Simultaneous determination of rosuvastatin and atorvastatin in human serum using RP-HPLC/UV detection: Method development, validation and optimization of various experimental parameters. Journal of Chromatography B, 879 (9-10), 557–563. doi: https://doi.org/10.1016/j.jchromb.2011.01.004
- Vittal, S., Shitut, N. R., Kumar, T. R., Vinu, M. C. A., Mullangi, R., Srinivas, N. R. (2006). Simultaneous quantitation of rosuvastatin and gemfibrozil in human plasma by high-performance liquid chromatography and its application to a pharmacokinetic study. Biomedical Chromatography, 20 (11), 1252–1259. doi: https://doi.org/10.1002/bmc.692
- Gomes, F. P., García, P. L., Porto Alves, J. M., Singh, A. K., Kedor-Hackmann, E. R. M., Miritello Santoro, M. I. R. (2009). Development and Validation of Stability-Indicating HPLC Methods for Quantitative Determination of Pravastatin, Fluvastatin, Atorvastatin, and Rosuvastatin in Pharmaceuticals. Analytical Letters, 42 (12), 1784–1804. doi: https://doi.org/10.1080/00032710903060669
- Derzhavna farmakopeia Ukrainy. Vol. 1 (2015). Kharkiv: DP «Ukrainskyi naukovyi tsentr yakosti likarskykh zasobiv», 1128.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Liudmyla Halka, Tetyana Kucher, Liubomyr Kryskiw, Marjan Piponsk, Iryna Furdela, Tetyana Uglyar, Olha Poliak, Liliya Logoyda

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






