Study of the influence of ingredients on biopharmaceutical factors and pharmacological activity of a medicinal product with carrot extract and rutin
DOI:
https://doi.org/10.15587/2519-4852.2023.277562Keywords:
thick extract of carrot roots, rutin, technology, analysis, proctological diseasesAbstract
Aim. The work aimed to study the influence of the components of a soft rectal medicine with carrot root extract and rutin on biopharmaceutical parameters and its pharmacological activity.
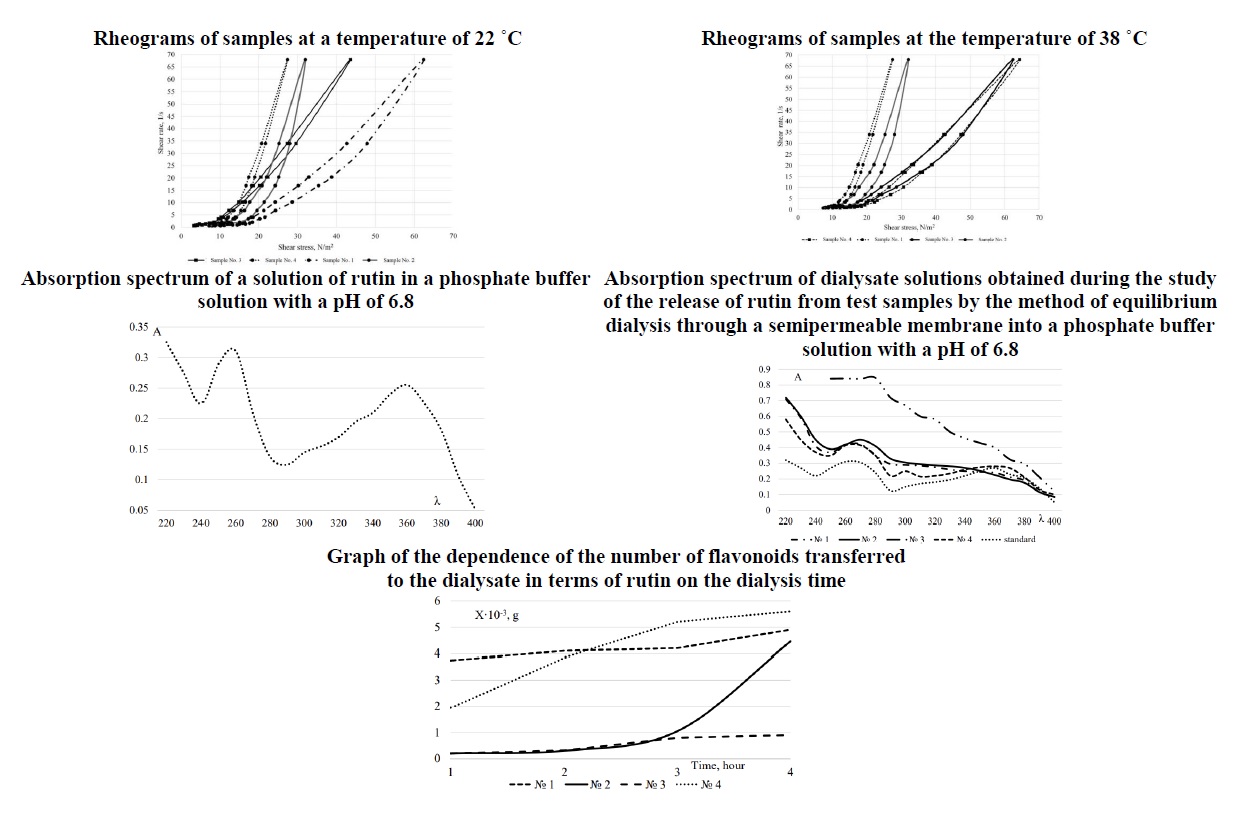
Materials and methods. The objects of the study were samples of soft pharmaceutical forms made on different bases. Pharmacological, biopharmaceutical, physicochemical and pharmacotechnological research methods were used in the study.
The results. According to the data of organoleptic studies, determination of colloidal stability, and determination of pH, it was established that the studied samples were stable during the entire observation period. According to the data of rheological studies, it was established that all systems are thixotropic. However, the recovery time of the system is different, which is related to the physicochemical properties of auxiliary substances included in the samples. The performed spectral analysis of dialysate solutions of experimental samples of soft medicine indicates the possibility of quantitative determination of the number of flavonoids in dialysates in terms of rutin. The components of the base of the samples and the thick extract of carrot roots do not interfere with the determination of rutin in dialysates with pH 6.8 by the absorption spectrophotometry method at a wavelength of 352 nm. The analysis of the obtained results of the study of the release of rutin from samples into a phosphate buffer solution by dialysis through a semipermeable membrane shows that the complete release is provided by auxiliary substances used in the preparation of sample No. 4, which is an emulsion of the first kind. The obtained data from pharmacological studies on the dynamics of planimetric indicators on the model of stencil wounds in rats demonstrated a wound-healing effect in all the studied samples and the reference agent - Hemorol suppositories. However, using sample No. 4 in the treatment of a stencil wound promotes faster healing, which in clinical use can contribute to reducing the risk of infection, the spread of infection, and reducing the area of the wound defect.
Conclusions. According to the results of the complex studies, moderate advantages of sample No. 4 over the comparison drug and other samples have been established, determining the perspective of further research
References
- Indrayan, A., Malhotra, K. R. (2018). Medical biostatistics. Boca Raton: CRC Press, 685.
- Tol, R. R., Kleijnen, J., Watson, A. J. M., Jongen, J., Altomare, D. F., Qvist, N., Higuero, T., Muris, J. W. M., Breukink, S. O., Henquet, C. J. M. (2020). European Society of ColoProctology: guideline for haemorrhoidal disease. Colorectal Disease, 22 (6), 650–662. doi: https://doi.org/10.1111/codi.14975
- Lohsiriwat, V. (2015). Treatment of hemorrhoids: A coloproctologist’s view. World Journal of Gastroenterology, 21 (31), 9245–9252. doi: https://doi.org/10.3748/wjg.v21.i31.9245
- Zaychenko, V. S., Ruban, О. А., Khanin, V. A., Kyslychenko, V. S., Masliy, Ju. S. (2018). Development of meloxicam and indole-3-carbinol quantification method in rectal suppositories for prevention and treatment of benign diseases of the prostate gland. International Journal of Green Pharmacy, 12 (2), 129–135.
- Velia, M., Ruban, O., Khalavka, M., Hohlova, L. (2021). Research of the choice of the basis of a semi-solid medicine with a semi-solid extract of Feverfew (Tanacetum parthenium). ScienceRise: Pharmaceutical Science, 1 (29), 51–59. doi: https://doi.org/10.15587/2519-4852.2021.225764
- Borko, Y., Inna, K., Grudko, V., Kononenko, N., Velya, M. (2022). Comprehensive study for the development of rectal suppositories with diosmin and hesperidin. ScienceRise: Pharmaceutical Science, 1 (35), 14–21. doi: https://doi.org/10.15587/2519-4852.2022.253518
- Pata, F., Gallo, G., Pellino, G., Vigorita, V., Podda, M., Di Saverio, S. et al. (2021). Evolution of Surgical Management of Hemorrhoidal Disease: An Historical Overview. Frontiers in Surgery, 8. doi: https://doi.org/10.3389/fsurg.2021.727059
- Sheikh, P., Lohsiriwat, V., Shelygin, Y. (2020). Micronized Purified Flavonoid Fraction in Hemorrhoid Disease: A Systematic Review and Meta-Analysis. Advances in Therapy, 37 (6), 2792–2812. doi: https://doi.org/10.1007/s12325-020-01353-7
- Ahmadi, Z., Mohammadinejad, R., Roomiani, S., Afshar, E. G., Ashrafizadeh, M. (2021). Biological and Therapeutic Effects of Troxerutin: Molecular Signaling Pathways Come into View. Journal of Pharmacopuncture, 24 (1), 1–13. doi: https://doi.org/10.3831/kpi.2021.24.1.1
- Godeberge, P., Sheikh, P., Lohsiriwat, V., Jalife, A., Shelygin, Y. (2021). Micronized purified flavonoid fraction in the treatment of hemorrhoidal disease. Journal of Comparative Effectiveness Research, 10 (10), 801–813. doi: https://doi.org/10.2217/cer-2021-0038
- Ju, L., Ke, F., Yadav, P. (2012). Herbal medicine in the treatment of ulcerative colitis. Saudi Journal of Gastroenterology, 18 (1), 3–10. doi: https://doi.org/10.4103/1319-3767.91726
- Falzon, C. C., Balabanova, A. (2017). Phytotherapy: An Introduction to Herbal Medicine. Primary Care: Clinics in Office Practice, 44 (2), 217–227. doi: https://doi.org/10.1016/j.pop.2017.02.001
- Stadnytska, N. Ye., Pavliuk, I. V., Dumych, I. I., Blonskyi, O. V. (2014). Doslidzhennia perspektyvnosti vykorystannia plodiv morkvy dykoi yak dzherela novykh kompleksiv biolohichno aktyvnykh rechovyn. Visnyk Natsionalnoho universytetu "Lvivska politekhnika". Khimiia, tekhnolohiia rechovyn ta yikh zastosuvannia, 787, 243–248.
- Pazyuk, D.-M. V., Zhuravel, I. A., Kyslychenko, A. A., Burda, N. Ye., Korniyenko, S. I., Mogilnaya, E. N. (2017). HPLC determination of phenolic acids in the underground part of carrots of “Nantska Kharkivska” and “Yaskrava” varieties. Research Journal of Pharmaceutical, Biological and Chemical Sciences, 8 (2), 1833–1836.
- Ayeni, E. A., Abubakar, A., Ibrahim, G., Atinga, V., Muhammad, Z. (2018). Phytochemical, nutraceutical and antioxidant studies of the aerial parts of Daucus carota L.(Apiaceae). Journal of Herbmed Pharmacology, 7 (2), 68–73. doi: https://doi.org/10.15171/jhp.2018.12
- Sheila, J., Priyadarshini, S., Sarah, J. M., Arumugam, P. (2017). Phytochemical profile and thin layer chromatographic studies of Daucus carota peel extracts. International Journal of Food Science and Nutrition, 2 (1), 23–26.
- Shebaby, W. N., Daher, C. F., El-Sibai, M., Bodman-Smith, K., Mansour, A., Karam, M. C., Mroueh, M. (2015). Antioxidant and hepatoprotective activities of the oil fractions from wild carrot (Daucus carotassp.carota). Pharmaceutical Biology, 53 (9), 1285–1294. doi: https://doi.org/10.3109/13880209.2014.976349
- Yarnykh, T. G., Ivaniuk, O. I., Kovalevska, I. V., Kukhtenko, H. P., Kutsenko, S. A. (2018). Rheology-based substantiation of a gel-former choice for vaginal gel. Journal of Pharmaceutical Sciences and Researc, 1 (11), 2825–2828.
- State Pharmacopoeia of Ukraine. Vol. 1 (2015). Kharkiv: RIREG, State Enterprise "Scientific Expert Pharmacopoeia Center", 1135.
- Maslii, Y., Ruban, O., Kasparaviciene, G., Kalveniene, Z., Materiienko, A., Ivanauskas, L. et al. (2020). The Influence of pH Values on the Rheological, Textural and Release Properties of Carbomer Polacril® 40P-Based Dental Gel Formulation with Plant-Derived and Synthetic Active Components. Molecules, 25 (21), 5018. doi: https://doi.org/10.3390/molecules25215018
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes (2010). OJEU, L276, 33–79.
- Kovalenka, V. M. (Ed.) (2019). Compendium 2019 – medicinal products. Kyiv: MORION, 2480.
- Kyslychenko O. A., Protska V. V., Zhuravel I. O., Hutsol V. V. (2018). The study of Daucus carota subsp. sativus fruits fatty acid composition of «Olenka», «Kharkivska Nantska» and «Yaskrava» varieties. Research Journal of Pharmaceutical, Biological and Chemical Sciences, 9 (6), 307–312.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Olena Ruban, Mohammad Al Sayasneh, Inna Kovalevska , Volodymir Grudko , Dmytro Lytkin, Oksana Dunaіevska

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






