Development and validation of a new spectrophotometric method for the determination of gabapentin in capsules
DOI:
https://doi.org/10.15587/2519-4852.2023.283270Keywords:
spectrophotometry, gabapentin, diazole red 2J, validation studies, SPhUAbstract
In Ukraine, there are about 100 thousand people with epilepsy. Gabapentin is an effective antiepileptic agent for oral use, presented in capsules of different dosages from different manufacturers. Therefore, the urgent task of pharmaceutical analysis today is the development of highly accurate, reliable, affordable and fast methods of quantitative determination.
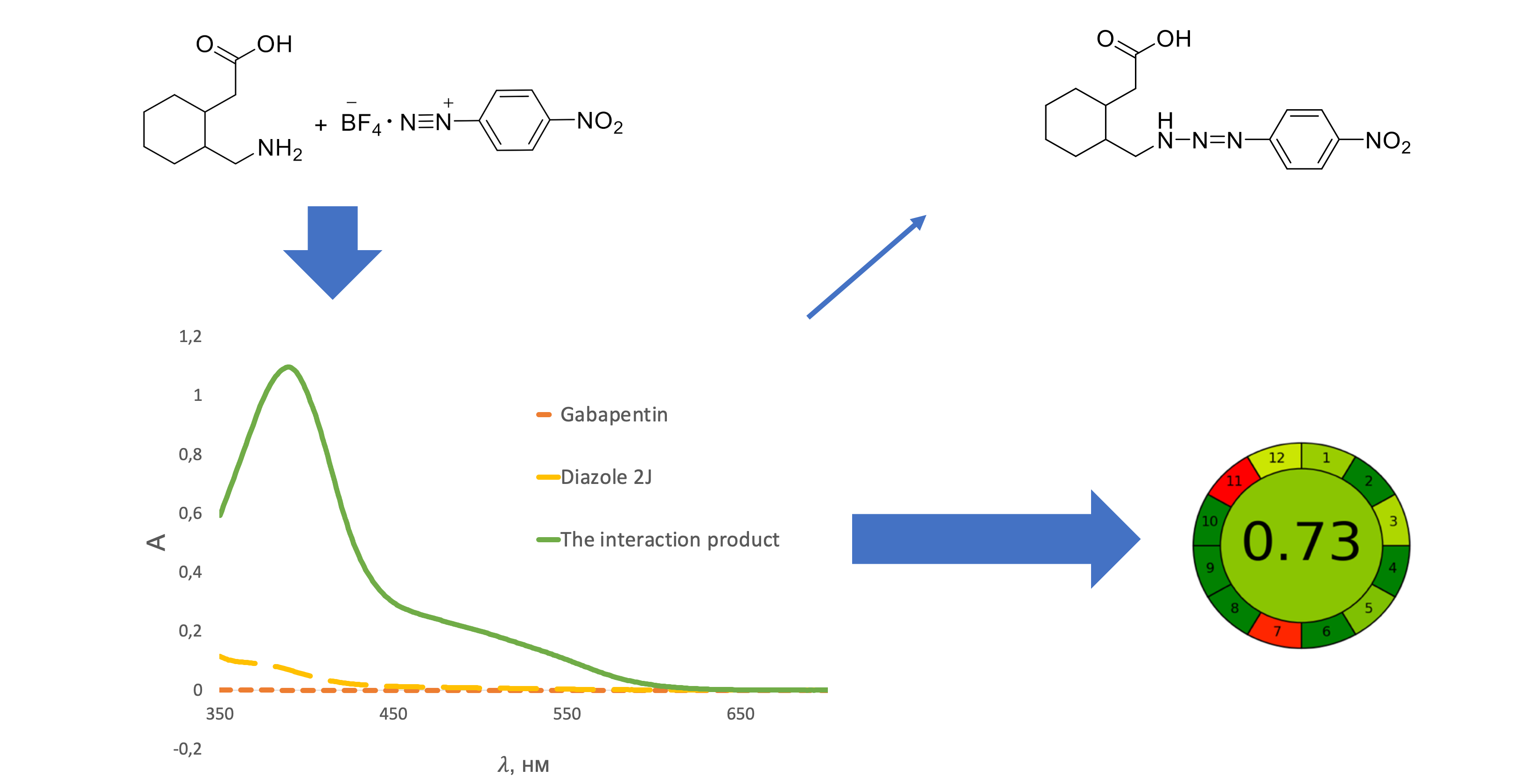
The aim of the work is to develop a spectrophotometric technique for the quantitative determination of gabapentin in capsules based on the reaction with diazole red 2J in compliance with the SPhU.
Material and methods. As reagent and solvent, diazole red 2J of AR grade, acetone of AR grade and purified water was used. Analytical equipment: Specord 200 and Specord 250 Plus spectrophotometers, ABT-120-5DM and Radwag XA 210.4Y electronic scales, Elmasonic E 60H and Sonorex Digitec DT100H ultrasonic baths, measuring glassware of A class.
Results. A simple, accurate and eco-friendly colourimetric method was developed for the quantification of gabapentin in capsules. The method was based on the reaction of gabapentin with diazole red 2J to give a coloured product having analytical maxima at 390 nm. Factors affecting colour development and stability were optimized and incorporated into the procedure. Regression analysis of Beer's plot showed a good correlation (not less than 0.999) in a concentration range of 2.10 – 3.64 mg/100 ml. The detection and quantification limits were 2.25 % and 6.19 %, respectively. The intra- and inter-laboratory precision demonstrates and reflects no interference by the capsule additives and confirms the reproducibility of the method in the selected concentration range. The prediction of the total uncertainty of the results of the developed method is calculated and displayed to assess the correctness of the reproduction of the method.
Conclusions. It has been proven that the developed method meets the requirements of the State Pharmacopoeia of Ukraine and allows to perform the correct quality control of medicinal products.
References
- Patsalos, P. N., Spencer, E. P., Berry, D. J. (2018). Therapeutic Drug Monitoring of Antiepileptic Drugs in Epilepsy: A 2018 Update. Therapeutic Drug Monitoring, 40 (5), 526–548. doi: https://doi.org/10.1097/ftd.0000000000000546
- Abdulrahman, S., Basavaiah, K. (2011). Non-aqueous titrimetric assay of gabapentin in capsules using perchloric acid as titrant. Chemical Industry and Chemical Engineering Quarterly, 17 (2), 173–178. doi: https://doi.org/10.2298/ciceq101011001a
- Sayed, R., El-Masry, M., Hassan, W., El-Mammli, M., Shalaby, A., Aboul-Enein, H. Y. (2018). Validated HPLC Method for the simultaneous determination of acyclovir and co-administered vitamin B3and gabapentin in spiked human plasma. Separation Science Plus, 1 (7), 475–482. doi: https://doi.org/10.1002/sscp.201800040
- Desireddy, R. B., Jitendra Kumar, P., Naga Sowjanya, G., Prachet, P., Vijay Kumar, Ch., Suresh Kumar, G., Srinivas Rao, K. (2012). Development and validation of RP-HPLC method for quantitative analysis of gabapentin in pure and pharmaceutical formulations. International Journal of Chemical Science, 10 (4), 2209–2217.
- Martinc, B., Roškar, R., Grabnar, I., Vovk, T. (2014). Simultaneous determination of gabapentin, pregabalin, vigabatrin, and topiramate in plasma by HPLC with fluorescence detection. Journal of Chromatography B, 962, 82–88. doi: https://doi.org/10.1016/j.jchromb.2014.05.030
- Sandhya, S. M., Jyothisree, G., Babu, G. (2014). Analysis of gabapentin by HPTLC with densitometric measurement after derivatization. International Journal of Pharmacy and Pharmaceutical Sciences, 6 (5), 707–710.
- Binaya, D. (2013). Estimation of gabapentin in human plasma using LC-MS/MS method. Asian Journal of Pharmaceutical and Clinical Research, 6 (3), 213–216.
- Naguib, I. A., Ali, N. A., Elroby, F. A., El Ghobashy, M. R., Abdallah, F. F. (2020). US FDA-validated green GC–MS method for analysis of gabapentin, tramadol and/or amitriptyline mixtures in biological fluids. Bioanalysis, 12 (21), 1521–1533. doi: https://doi.org/10.4155/bio-2020-0217
- Lin, X., Cai, Y., Yan, J., Zhang, L., Wu, D., Li, H. (2014). Determination of Gabapentin in Human Plasma and Urine by Capillary Electrophoresis with Laser-Induced Fluorescence Detection. Journal of Chromatographic Science, 53 (6), 986–992. doi: https://doi.org/10.1093/chromsci/bmu134
- Abd Alrassol, K. S., Mousa, M. N. (2019). Estimation and evaluation of gabapentin and pregabaline anti-epileptic drugs in bulk and pharmaceutical preparations by eco-friendly bromate-bromide reagent. Eurasian Journal of Analytical Chemistry, 14 (2), 10–20.
- Abd-Alrassol, K. S., Qasim, Q. A., Hassan Shari, F., AL-Salman, H. N. K., Hussein, H. H. (2020). The spectrophotometric determination of antiepileptic drug in standard and pharmaceutical formulations by diazotization coupling reaction and some metals complexes. Systematic Reviews in Pharmacy, 11 (3), 247–260.
- Abdellatef, H. E., Khalil, H. M. (2003). Colorimetric determination of gabapentin in pharmaceutical formulation. Journal of Pharmaceutical and Biomedical Analysis, 31 (1), 209–214. doi: https://doi.org/10.1016/s0731-7085(02)00572-1
- Mohammed, T. O., Elbashir, A. A. (2015). Spectrophotometric method for determination of gabapentin in pharmaceutical formulation by derivatization with 4-chloro-7-nitrobenzo-2-oxa-1,3-diazole (NBD-Cl). International Journal of Drug Development and Research, 7 (4), 1–4.
- Fonseca, F., Brito de Barros, R., Ilharco, L. M., Garcia, A. R. (2017). Spectroscopic Methods for Quantifying Gabapentin: Framing the Methods without Derivatization and Application to Different Pharmaceutical Formulations. Applied Spectroscopy, 71 (11), 2519–2531. doi: https://doi.org/10.1177/0003702817716181
- Abdal Rassol, K. S., Qasim, Q. A., AL-Salman, H. N. K. (2019). Spectral kinetic method and its applications in the evaluation of gabapentin. International Journal of Green Pharmacy, 12 (4), 36–42. doi: https://doi.org/10.22377/ijgp.v12i04.2208
- Miedviedieva, K., Vasyuk, S., Korzhova, A., Pavljuk, I. (2022). New, simple and express determination of lamotrigine in tablets by using diazole red 2J. ScienceRise: Pharmaceutical Science, 1 (35), 44–51. doi: https://doi.org/10.15587/2519-4852.2022.253542
- Hryzodub, A. Y. (2016). Standartyzovannыe protsedurы valydatsyy metodyk kontrolia kachestva lekarstvennыkh sredstv. Kharkiv: Ukrainskyi naukovyi farmakopeinyi tsentr yakosti likarskykh zasobiv, 396.
- Derzhavna Farmakopeia Ukrainy. Vol. 1 (2015). Kharkiv: Naukovo-ekspertnyi farmakopeinyi tsentr, 1128.
- Gałuszka, A., Migaszewski, Z. M., Konieczka, P., Namieśnik, J. (2012). Analytical Eco-Scale for assessing the greenness of analytical procedures. TrAC Trends in Analytical Chemistry, 37, 61–72. doi: https://doi.org/10.1016/j.trac.2012.03.013
- Pena-Pereira, F., Wojnowski, W., Tobiszewski, M. (2020). AGREE – Analytical GREEnness Metric Approach and Software. Analytical Chemistry, 92 (14), 10076–10082. doi: https://doi.org/10.1021/acs.analchem.0c01887
- Essam, H. M., Saad, M. N., Elzanfaly, E. S., Amer, S. M. (2021). Optimization and validation of Eco-friendly RP-HPLC and univariate spectrophotometric methods for the simultaneous determination of Fluorometholone and Tetrahydrozoline hydrochloride. Acta Chromatographica, 33 (3), 216–227. doi: https://doi.org/10.1556/1326.2020.00783
- Kazemipour, M., Fakhari, I., Ansari, M. (2013). Gabapentin Determination in Human Plasma and Capsule by Coupling of Solid Phase Extraction, Derivatization Reaction, and UV-Vis Spectrophotometry. Iranian Journal of Pharmaceutical Research, 12 (3), 247–253.
- Chandra, D. S., Desireddy, R. B., Jitendrakumar, P., Narisireddy, P. (2012). Development and Validation of UV spectrophotometric method for Estimation of Gabapentin in Pharmaceutical dosage form. International Journal of Chemical and Pharmaceutical Sciences, 3 (4), 61–63.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Kateryna Miedviedieva, Svitlana Vasyuk, Olena Portna

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






