Analysis of pharmaceutical supply of breast cancer patients
DOI:
https://doi.org/10.15587/2519-4852.2023.283490Keywords:
breast cancer, clinical and economic analysis, frequency analysis, ABC analysis, VEN analysis, pharmaceutical support, drugAbstract
The aim: to conduct a clinical and economic analysis of the state of pharmaceutical support for patients with breast cancer in women.
Materials and methods. During the research, data from the National List of Essential Medicines, the 14th State Formulary of Medicines, the Ukrainian clinical protocol, the clinical protocols of Great Britain and the United States of America for the treatment of breast cancer, and a depersonalized database of drug prescriptions were used. The research used such methods as clinical-economic, organizational-economic, mathematical-statistical, graphic, grouping and generalization.
Research results. It was established that 46 schemes of schemes for the treatment of breast cancer are presented in Ukrainian and international clinical protocols (Great Britain, USA). In all three protocols, there were 5 regimens for the treatment of breast cancer, such as CMF (Cyclophosphamide, Methotrexat, Fluorouracil), AC (Doxorubicin, Cyclophosphamide), DC (Docetaxel, Cyclophosphamide), TC (Trastuzumab, Capecitabin), EC (Epirubicin, Cyclophosphamide).
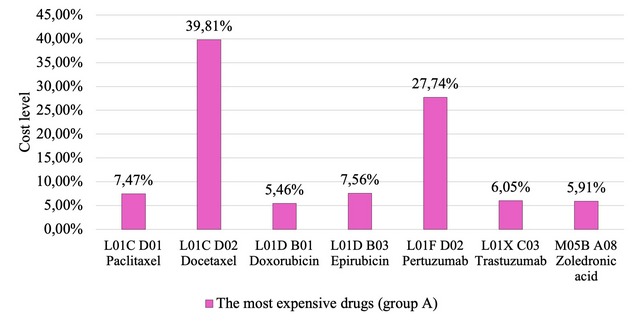
Analysis of the pharmaceutical component in the protocols for individual drugs showed that they included 23 drugs according to the INN, which belong to the group of antineoplastic drugs. The Ukrainian clinical protocol includes 17 drugs, the British protocol – 14 drugs, and the American one – 15 drugs. The frequency analysis of antineoplastic drugs included in the Ukrainian clinical protocol revealed the TOP-3 in terms of drug prescription frequency: L01A A01 Cyclophosphamide (Ki=0.037), L01C D02 Docetaxel (Ki=0.029), L01D B01 Doxorubicin (Ki=0.026). The largest number of prescribed drugs are vital drugs – 61.96 %, the smallest share – essential drugs – 2.17 %, and the share of non-essential drugs is 35.87 %. The ABC analysis made it possible to determine the group of the most expensive drugs in the treatment of breast cancer - these are antineoplastic and immunomodulating agents. The total expenses for this group amounted to UAH 772,459.96, which according to the US dollar exchange rate of the National Bank of Ukraine at the end of August 2022 was USD 21,122.77. According to the analysis of the matrix of the integrated ABC-VEN-frequency analysis, it was established that the largest number of prescriptions belonged to the group of less expensive and vital drugs (B/V) – 7 drugs (48.73 % of the total amount. At the same time, the largest amount of expenses is observed in for the A/V group – UAH 772,459.96 (52.83 % of all costs directed to the pharmaceutical support of patients). The results of the structural analysis of the group of the most expensive drugs showed that the highest costs were characteristic of L01CD02 Docetaxel (UAH 1166,531.31; 39.81 % of the total cost in the group), and the lowest – for L01DB01 Doxorubicin – UAH 63,694.14 (5.46 %, respectively).
Conclusions. According to the results of the conducted research, it was established that the largest costs fall on the group of essential drugs (1047735.07 UAH, 71.65 % of all costs). This determines the need for further pharmacoeconomic calculations of breast cancer treatment to optimize the costs of antineoplastic drugs
References
- International Agency for Research on Cancer. World Health Organization. Available at: https://gco.iarc.fr/today/data/factsheets/populations/900-world-fact-sheets.pdf
- International Agency for Research on Cancer. World Health Organization. Available at: https://gco.iarc.fr/today/data/factsheets/populations/804-ukraine-fact-sheets.pdf
- Arnold, M., Morgan, E., Rumgay, H., Mafra, A., Singh, D., Laversanne, M. et al. (2022). Current and future burden of breast cancer: Global statistics for 2020 and 2040. The Breast, 66, 15–23. doi: https://doi.org/10.1016/j.breast.2022.08.010
- Semin, J. N., Palm, D., Smith, L. M., Ruttle, S. (2020). Understanding breast cancer survivors’ financial burden and distress after financial assistance. Supportive Care in Cancer, 28 (9), 4241–4248. doi: https://doi.org/10.1007/s00520-019-05271-5
- Greenup, R. A., Rushing, C., Fish, L., Campbell, B. M., Tolnitch, L., Hyslop, T. et al. (2019). Financial Costs and Burden Related to Decisions for Breast Cancer Surgery. Journal of Oncology Practice, 15 (8), e666–e676. doi: https://doi.org/10.1200/jop.18.00796
- Cancer Ukraine 2020 country profile. World Health Organization. Available at: https://cdn.who.int/media/docs/default-source/country-profiles/cancer/ukr-2020.pdf?sfvrsn=5d341f5f_2&download=true
- European Region profile. World Health Organization. Available at: https://cdn.who.int/media/docs/default-source/ncds/ncd-surveillance/cancer-profiles-2020/euro-cancer-profile-2020.pdf?sfvrsn=6fbcb00e_3
- Pikhotska, O., Khomiakova, I. (2021). Financing of the healthcare system in the context of its reformation. Derzhavne upravlinnya: udoskonalennya ta rozvytok, 12. doi: https://doi.org/10.32702/2307-2156-2021.12.33
- Krynychko, L., Motailo, O. (2021). New approaches to financing the health care system. Public Administration Aspects, 9 (2), 86–100. doi: https://doi.org/10.15421/152122
- Deiaki pytannia realizatsii prohramy derzhavnykh harantii medychnoho obsluhovuvannia naselennia u 2022 rotsi (2021). Postanova Kabinetu Ministriv Ukrainy No. 1440. 29.12.2021. Available at: https://zakon.rada.gov.ua/laws/show/1440-2021-%D0%BF#Text
- Vymohy do «Prohramy medychnykh harantii – 2023». Available at: https://contracting.nszu.gov.ua/kontraktuvannya/kontraktuvannya-2023/vimogi-pmg-2023
- Pro dohovory pro medychne obsluhovuvannia naselennia za prohramoiu medychnykh harantii (2018). Postanova Kabinetu Ministriv Ukrainy No. 410. 25.04.2018. Available at: https://zakon.rada.gov.ua/laws/show/410-2018-%D0%BF#Text
- Deiaki pytannia derzhavnoho rehuliuvannia tsin na likarski zasoby i vyroby medychnoho pryznachennia (2009). Postanova KMU No. 333. 25.03.2009. Available at: https://zakon.rada.gov.ua/laws/show/333-2009-%D0%BF#n15
- Pro zatverdzhennia ta vprovadzhennia medyko-tekhnolohichnykh dokumentiv zi standartyzatsii medychnoi dopomohy pry raku molochnoi zalozy (2015). Nakaz MOZ Ukrainy No. 396. 30.06.2015. Available at: https://www.dec.gov.ua/wp-content/uploads/2019/11/2015_396_ykpmd_rmz.pdf
- Pro zatverdzhennia chotyrnadtsiatoho vypusku Derzhavnoho formuliara likarskykh zasobiv ta zabezpechennia yoho dostupnosti (2022). Nakaz MOZ Ukrainy No. 1011. 13.06.2022. Available at: https://moz.gov.ua/uploads/ckeditor/документи/dn_1011_13.06.2022_dod.pdf
- Clinical Guidelines for the Management of Breast Cancer. Available at: https://www.england.nhs.uk/mids-east/wp-content/uploads/sites/7/2018/02/guidelines-for-the-management-of-breast-cancer-v1.pdf
- NCCN Clinical Practice Guidelines in Oncology. Breast Cancer (2023). NCCN Evidence BlocksTM. Version 4. Available at: https://www.nccn.org/professionals/physician_gls/pdf/breast_blocks.pdf
- Zaliska, O. M., Stasiv, Kh.-O. J. (2019). Scientific methodology and practical use of managed entry agreements for innovative medicines in system of health technology assessment in Ukraine. Farmatsevtychnyi Zhurnal, 4, 32–40. doi: https://doi.org/10.32352/0367-3057.4.19.04
- Nemchenko, A. S., Nazarkina, V. M., Kosiachenko, K. L., Babenko, M. M. (2023). Problems of forming a professional environment in the health technology assessment system in Ukraine. Health & Education, 2, 28–36. doi: https://doi.org/10.32782/health-2023.2.5
- Fang, J.-Q. (Ed.) (2017). Handbook of Medical Statistics. Sun Yat-Sen University, 852.
- Harding, J. J., Piha-Paul, S. A., Shah, R. H., Murphy, J. J., Cleary, J. M., Shapiro, G. I. et al. (2023). Antitumour activity of neratinib in patients with HER2-mutant advanced biliary tract cancers. Nature Communications, 14 (1). doi: https://doi.org/10.1038/s41467-023-36399-y
- Senkus, E., Delaloge, S., Domchek, S. M., Conte, P., Im, S., Xu, B. et al. (2023). Olaparib efficacy in patients with germline BRCA mutated, HER2 negative metastatic breast cancer: Subgroup analyses from the phase III OlympiAD trial. International Journal of Cancer, 153 (4), 803–814. doi: https://doi.org/10.1002/ijc.34525
- Smyth, L. M., Tamura, K., Oliveira, M., Ciruelos, E. M., Mayer, I. A., Sablin, M.-P. et al. (2020). Capivasertib, an AKT Kinase Inhibitor, as Monotherapy or in Combination with Fulvestrant in Patients withAKT1E17K-Mutant, ER-Positive Metastatic Breast Cancer. Clinical Cancer Research, 26 (15), 3947–3957. doi: https://doi.org/10.1158/1078-0432.ccr-19-3953
- Saesen, R., Lacombe, D., Huys, I. (2021). Design, organisation and impact of treatment optimisation studies in breast, lung and colorectal cancer: The experience of the European Organisation for Research and Treatment of Cancer. European Journal of Cancer, 151, 221–232. doi: https://doi.org/10.1016/j.ejca.2021.04.012
- Pokaznyk serednoi zarobitnoi platy za 2022 rik v Ukraini (2023). Available at: https://www.pfu.gov.ua/2152284-pokaznyk-serednoyi-zarobitnoyi-platy-za-2022-rik/
- Visscher, B. B., Vervloet, M., te Paske, R., van Dijk, L., Heerdink, E. R., Rademakers, J. (2021). Implementation of an animated medication information tool in community pharmacies, with a special focus on patients with limited health literacy. International Journal of Pharmacy Practice, 29 (6), 566–572. doi: https://doi.org/10.1093/ijpp/riab038
- Solà-Morales, O., Volmer, T., Mantovani, L. (2019). Perspectives to mitigate payer uncertainty in health technology assessment of novel oncology drugs. Journal of Market Access & Health Policy, 7 (1), 1562861. doi: https://doi.org/10.1080/20016689.2018.1562861
- Jönsson, B., Hampson, G., Michaels, J., Towse, A., von der Schulenburg, J.-M. G., Wong, O. (2018). Advanced therapy medicinal products and health technology assessment principles and practices for value-based and sustainable healthcare. The European Journal of Health Economics, 20 (3), 427–438. doi: https://doi.org/10.1007/s10198-018-1007-x
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Yaroslava Rafalska, Kostyantyn Kosyachenko

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






