Substantiation of creation of transdermal forms of drug delivery with antihypertensive action
DOI:
https://doi.org/10.15587/2519-4852.2023.286303Keywords:
arterial hypertension, transdermal therapeutic systems, ACE inhibitors, enalapril, lisinopril, captoprilAbstract
The article presents the theoretical justification for the choice of active pharmaceutical ingredients of antihypertensive action for the creation of new forms of delivery - transdermal therapeutic systems.
Purpose: monitoring of studies on the use of angiotensin-converting enzyme (ACE) inhibitors in the development of transdermal drugs for the treatment of hypertension.
Materials: open access electronic resources of scientific periodicals from around the world: Google Scholar (https://scholar.google.com/); PubMed (https://pubmed.ncbi.nlm.nih.gov/); Sciencedirect (https://www.sciencedirect.com/); Pubchem (https://pubchem.ncbi.nlm.nih.gov/); National Library of Ukraine named after V. I. Vernadskyi (http://www.nbuv.gov.ua/); Ukrainian Institute of Intellectual Property (Ukrpatent) (https://sis.ukrpatent.org/uk/); Industrial Property (https://base.uipv.org/searchBul/); USPTO. The United States Patent and Trademark Office (https://www.uspto.gov/patents/search); European Patent Office (EPO) (https://www.epo.org/).
Methods of the research: information search, theoretical analysis and systematization of data from scientific sources, logical analysis.
Results: a review of scientific sources was carried out, and an analysis of ACE inhibitors as candidates for the creation of transdermal forms for the treatment of arterial hypertension was carried out. The pharmacological and physicochemical aspects of the possibility of their use for introduction through the skin are defined. It has been established that the search and development of the latest means of treatment of arterial hypertension, which would significantly increase the duration and quality of life of patients, are extremely relevant. Transdermal drug delivery systems are one of the pharmaceutical products being developed on the world market, and their use allows overcoming the associated disadvantages of other delivery routes.
Conclusions: the review of domestic and foreign literature confirmed the relevance of biopharmaceutical research in the field of development of innovative dosage forms - transdermal therapeutic systems for the treatment of hypertension.
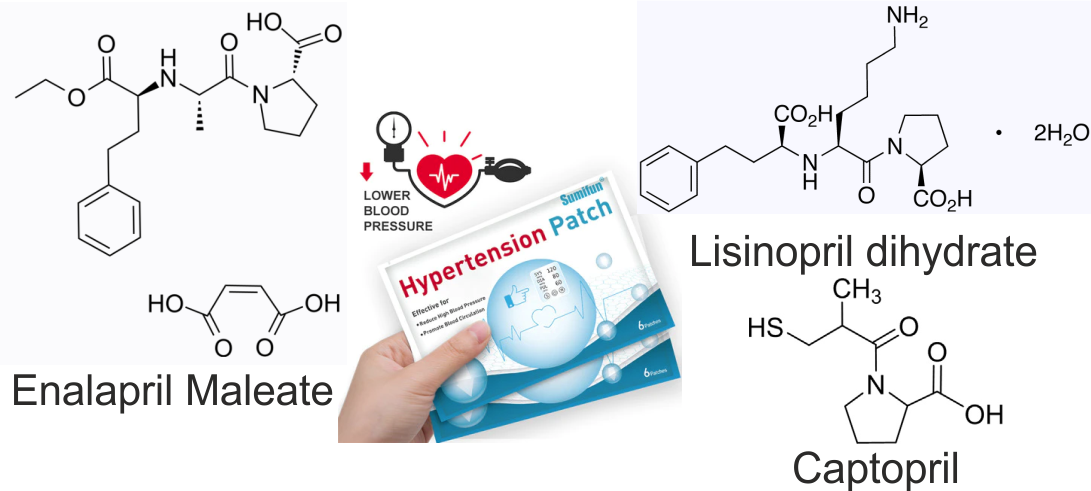
The choice of promising antihypertensive active pharmaceutical ingredients of the ACE group (enalapril maleate, lisinopril dihydrate, and captopril) is substantiated, taking into account their specific physicochemical properties that are suitable for penetration through the skin.
The market for transdermal drug delivery is increasing, and there is a prospect of higher growth rates in this market in the coming years
References
- Whelton, P. K., Carey, R. M., Aronow, W. S., Casey, D. E., Collins, K. J., Dennison Himmelfarb, C. et al. (2018). 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation, 138 (17), e426–e483. doi: https://doi.org/10.1161/cir.0000000000000597
- Mills, K. T., Stefanescu, A., He, J. (2020). The global epidemiology of hypertension. Nature Reviews Nephrology, 16 (4), 223–237. doi: https://doi.org/10.1038/s41581-019-0244-2
- Hypertension (2022). World Health Organization (WHO). Available at: https://www.who.int/health-topics/hypertension/#tab=tab_1
- Stierman, B., Afful, J., Carroll, M. D., Chen, T. et al. (2021). National Health and Nutrition Examination Survey 2017 – March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes. National Center for Health Statistics (U.S.), NHSR No. 158. Available at: https://stacks.cdc.gov/view/cdc/106273
- Marushko, Yu.V., Hyshchak, T.V. (2018). Arterialna hipertenziia u ditei ta pidlitkiv. Zdorov’ia Ukrainy. Pediatriia, 4 (47), 53–58. Available at: https://health-ua.com/multimedia/3/9/2/3/5/1547021303.pdf
- Shlimkevych, I. V., Lembryk, I. S., Tsytsiura, O. O., Alekseieva, Yu. I., Zhyliak, O. V. (2022). Arterial hypertension in children and adolescents: a modern view at the problem (a literature review). Part 1. Zaporozhye Medical Journal, 24 (2), 248–253. doi: https://doi.org/10.14739/2310-1210.2022.2.235489
- Senatorova, H. S., Honchar, M. O., Alenina, I. S. et al.; Senatorova, H. S. (Ed.) (2018). Arterialna hipertenziia u ditei. Kharkiv: PLANETA-PRYNT, 108.
- Vsesvitnii den borotby z arterialnoiu hipertenziieiu. Derzhavna ustanova «Tsentr hromadskoho zdorov’ia Ministerstva okhorony zdorov’ia Ukrainy». Available at: https://www.phc.org.ua/news/vsesvitniy-den-borotbi-z-arterialnoyu-gipertenzieyu
- Kumar, G., Virmani, T., Pathak, K., Alhalmi, A. (2022). A Revolutionary Blueprint for Mitigation of Hypertension via Nanoemulsion. BioMed Research International, 2022, 1–12. doi: https://doi.org/10.1155/2022/4109874
- Li, C., Wang, J., Wang, Y., Gao, H., Wei, G., Huang, Y. et al. (2019). Recent progress in drug delivery. Acta Pharmaceutica Sinica B, 9 (6), 1145–1162. doi: https://doi.org/10.1016/j.apsb.2019.08.003
- Bird, D., Ravindra, N. M. (2020). Transdermal drug delivery and patches – An overview. Medical Devices & Sensors, 3 (6). doi: https://doi.org/10.1002/mds3.10069
- Zhao, Z., Ukidve, A., Kim, J., Mitragotri, S. (2020). Targeting Strategies for Tissue-Specific Drug Delivery. Cell, 181 (1), 151–167. doi: https://doi.org/10.1016/j.cell.2020.02.001
- Lee, H., Song, C., Baik, S., Kim, D., Hyeon, T., Kim, D.-H. (2018). Device-assisted transdermal drug delivery. Advanced Drug Delivery Reviews, 127, 35–45. doi: https://doi.org/10.1016/j.addr.2017.08.009
- Steven, J. M. (2019). Press Release Transdermal Drug Delivery Market – Global Drivers, Restraints, Opportunities, Trends, and Forecasts, 2017–2023.
- Ramadon, D., McCrudden, M. T. C., Courtenay, A. J., Donnelly, R. F. (2021). Enhancement strategies for transdermal drug delivery systems: current trends and applications. Drug Delivery and Translational Research, 12 (4), 758–791. doi: https://doi.org/10.1007/s13346-021-00909-6
- Guo, D., Dou, D., Li, X., Zhang, Q., Bhutto, Z. A., Wang, L. (2017). Ivermection-loaded solid lipid nanoparticles: preparation, characterisation, stability and transdermal behaviour. Artificial Cells, Nanomedicine, and Biotechnology, 46 (2), 255–262. doi: https://doi.org/10.1080/21691401.2017.1307207
- Vargason, A. M., Anselmo, A. C., Mitragotri, S. (2021). The evolution of commercial drug delivery technologies. Nature Biomedical Engineering, 5 (9), 951–967. doi: https://doi.org/10.1038/s41551-021-00698-w
- Facts About Hypertension (2022). Centers for Disease Control and Prevention. Atlanta: Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/bloodpressure/facts.htm
- Vsesvitnii den borotby z arterialnoiu hipertenziieiu (2022). Available at: https://www.oblses.ck.ua/index.php?view=article&catid=41%3A2013-05-13-02-14-47&id=2898%3Avsesvitnii-den-borotby-z-arterialnoiu-hipertenziieiu&format=pdf&option=com_content&Itemid=57
- Khyts, A. (2021). Suchasni mozhlyvosti likuvannia arterialnoi hipertenzii: fokus na ostanni rekomendatsii ISH 2020. Ukrainskyi medychnyi chasopys. Available at: https://www.umj.com.ua/article/208823/suchasni-mozhlivosti-likuvannya-arterialnoyi-gipertenziyi-fokus-na-ostanni-rekomendatsiyi-ish-2020
- Korol, S. V. (2021). Arterialna hipertenziia: ohliad suchasnykh rekomendatsii. Zdorov’ia Ukrainy 21 storichchia, 11-12 (504-505). Available at: https://health-ua.com/multimedia/6/6/2/4/2/1626685365.pdf
- Guideline for the pharmacological treatment of hypertension in adults (2021). Geneva: World Health Organization. Available at: https://apps.who.int/iris/bitstream/handle/10665/344424/9789240033986-eng.pdf
- Kulkarni, S., Graggaber, J. (2022). How to improve compliance to hypertension treatment. European Society of Cardiology, 22 (6). Available at: https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-22/how-to-improve-compliance-to-hypertension-treatment
- Mishchenko, L. A., Hulkevych, O. V. (2021). Nekontrolovana arterialna hipertenziia: dodatkovi mozhlyvosti pokrashchennia arterialnoho tysku ta yakosti zhyttia patsiientiv. Zdorov’ia Ukrainy 21 storichchia, 6 (499), 45–47. Available at: https://health-ua.com/multimedia/6/4/7/0/4/1619439395.pdf
- Unger, T., Borghi, C., Charchar, F., Khan, N. A., Poulter, N. R., Prabhakaran, D. et al. (2020). 2020 International Society of Hypertension global hypertension practice guidelines. Journal of Hypertension, 38 (6), 982–1004. doi: https://doi.org/10.1097/hjh.0000000000002453
- Chen, Y. J., Li, L. J., Tang, W. L., Song, J. Y., Qiu, R., Li, Q. et al. (2018). First-line drugs inhibiting the renin angiotensin system versus other first-line antihypertensive drug classes for hypertension. Cochrane Database of Systematic Reviews, 2018 (11). doi: https://doi.org/10.1002/14651858.cd008170.pub3
- Herman, L. L., Padala, S. A., Ahmed, I., Bashir, K. (2022). Angiotensin Converting Enzyme Inhibitors (ACEI). StatPearls. Treasure Island (FL). Available at: https://www.ncbi.nlm.nih.gov/books/NBK431051/
- Goyal, A., Cusick, A. S., Thielemier, B. (2022). ACE Inhibitors. StatPearls. Treasure Island. Available at: https://www.ncbi.nlm.nih.gov/books/NBK430896/
- Fountain, J. H., Lappin, S. L. (2022). Physiology, Renin Angiotensin System. StatPearls. NCBI Bookshelf version. StatPearls Publishing.
- Angiotensin Converting Enzyme (ACE) Inhibitors. Clinical Pharmacology (2021). Elsevier.
- Sirenko, Yu. M., Radchenko, H. D. (2004). Rol inhibitoriv anhiotenzynperetvoriuiuchoho fermentu v suchasnomu likuvanni khvorykh z arterialnoiu hipertenziieiu. Ukraynskyi kardyolohycheskyi zhurnal.
- Emilie, W. (2022). ACE Inhibitors: A Class and Utilization Review. Cardiology ADVISOR. Available at: https://www.thecardiologyadvisor.com/ddi/ace-inhibitors/
- Sirenko, Yu. M. (2006). Rol inhibitoriv anhiotenzynperetvoriuiuchoho fermentu v suchasnomu likuvanni sertsevo-sudynnykh zakhvoriuvan. Ratsionalna farmakoterapiia, 1 (1). Available at: https://rpht.com.ua/ua/archive/2006/1%281%29/article-4/rol-ingibitoriv-angiotenzinperetvoryuyuchogo-fermentu-v-suchasnomu-likuvanni-sercevo-sudinnih-zahvoryuvan#
- Rohman, M. S., Fajar, J. K., Kuncahyo, B. H., Yunita, L., Sidarta, E. P., Saka, P. N. B. et al. (2018). Angiotensin-converting enzyme (ACE) I/D and bradykinin B2 receptor T/C genes polymorphism in patients with ACE inhibitors-related cough. Egyptian Journal of Medical Human Genetics, 19 (4), 307–313. doi: https://doi.org/10.1016/j.ejmhg.2018.05.006
- Rahman, M., Pressel. S., Davis, B. R., Nwachuku, C. et al. (2005). Renal outcomes in high-risk hypertensive patients treated with an angiotensin-converting enzyme inhibitor or a calcium channel blocker vs a diuretic: a report from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Archives of Internal Medicine, 165 (8), 936–946. doi: https://doi.org/10.1001/archinte.165.8.936
- Anthony, A. B. (2016). Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial – ALLHAT. American College of Cardiology. Available at: https://www.acc.org/latest-in-cardiology/clinical-trials/2014/02/25/15/55/allhatnbsp8212nbsppresented-at-aha-2009
- Unger, T., Paulis, L., Sica, D. A. (2011). Therapeutic perspectives in hypertension: novel means for renin-angiotensin-aldosterone system modulation and emerging device-based approaches. European Heart Journal, 32 (22), 2739–2747. doi: https://doi.org/10.1093/eurheartj/ehr253
- Voronkov, L. H. (2007). Inhibitory APF ta adrenoblokatory u likuvanni khronichnoi sertsevoi nedostatnosti: klinichna rol ta metodolohiia zastosuvannia. Ratsionalna farmakoterapiia, 2 (3). Available at: https://rpht.com.ua/ua/archive/2007/2%283%29
- Choi, I. S., Park, I. B., Lee, K., Ahn, T. H., Kim, J. H., Ahn, Y. et al. (2018). Angiotensin-Converting Enzyme Inhibitors Provide Better Long-Term Survival Benefits to Patients With AMI Than Angiotensin II Receptor Blockers After Survival Hospital Discharge. Journal of Cardiovascular Pharmacology and Therapeutics, 24 (2), 120–129. doi: https://doi.org/10.1177/1074248418795897
- Enalapril: Uses, Interaction, Mechanism of Action (2022). DrugBank: a knowledgebase for drugs, drug actions and drug targets. Available at: https://go.drugbank.com/drugs/DB00584
- Lam, P. H., Packer, M., Fonarow, G. C., Faselis, C., Allman, R. M., Morgan, C. J., Singh, S. N., Pitt, B., Ahmed, A. (2020). Early Effects of Starting Doses of Enalapril in Patients with Chronic Heart Failure in the SOLVD Treatment Trial. The American Journal of Medicine, 133 (2), e25–e31. doi: https://doi.org/10.1016/j.amjmed.2019.06.053
- Еналаприл (Enalaprilum). Kompendium. Likarski preparaty. Available at: https://compendium.com.ua/dec/270895/#
- Olvera, L. E., Parmar, M., Pendela, V. S., Jamie M. Terrell (2022). Lisinopril. StatPearls. Treasure Island, StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK482230/
- Soloshenko, O. S., Serhyenko, O. M. (2011). Vibor preparata hruppi ynhybytorov APF: effektyvnost, bezopasnost y dostupnost dlia patsyenta. Ukrainskyi Medychnyi Chasopys. Likariu-praktyku, 1 (81), 60–64. Available at: https://www.umj.com.ua/article/9848/vybor-preparata-gruppy-ingibitorov-apf-effektivnost-bezopasnost-i-dostupnost-dlya-pacienta#list
- Lizynopryl (Lisinopril). Kompendium. Likarski preparaty. Available at: https://compendium.com.ua/uk/akt/76/3045/lisinoprilum/
- Obied, A. H. H., Ahmed, A. A. E. (2021). Evaluation of the clinical outcome of captopril use for hypertensive urgency in Khartoum State's emergency centres. African Journal of Emergency Medicine, 11 (1), 202–206. doi: https://doi.org/10.1016/j.afjem.2020.10.003
- Chekman, I. S., Horchakova, N. O., Kazak, L. I. et al.; Chekman, I. S. (Ed.) (2017). Farmakolohiia. Vinnytsia: Nova Knyha, 784.
- Kaptopryl (Captoprilum). Kompendium. Likarski preparaty. Available at: https://compendium.com.ua/akt/67/3102/captoprilum/#toc-0
- Park, S. (2019). Ideal Target Blood Pressure in Hypertension. Korean Circulation Journal, 49 (11), 1002–1009. doi: https://doi.org/10.4070/kcj.2019.0261
- Williams, B., Mancia, G., Spiering, W., Agabiti Rosei, E., Azizi, M., Burnier, M. et al. (2018). 2018 ESC/ESH Guidelines for the management of arterial hypertension. European Heart Journal, 39 (33), 3021–3104. doi: https://doi.org/10.1093/eurheartj/ehy339
- Abegaz, T. M., Shehab, A., Gebreyohannes, E. A., Bhagavathula, A. S., Elnour, A. A. (2017). Nonadherence to antihypertensive drugs: A systematic review and meta-analysis. Medicine (Baltimore), 96 (4), e5641. doi: https://doi.org/10.1097/md.0000000000005641
- Abbas, H., Hallit, S., Kurdi, M., Karam, R. (2022). Non-adherence to antihypertensive medications in Lebanese adults hospitalized for hypertensive urgency and its cost. BMC Cardiovascular Disorders, 22 (1). doi: https://doi.org/10.1186/s12872-022-02907-z
- Lane, D., Lawson, A., Burns, A., Azizi, M., Burnier, M., Jones, D. J. L. et al. (2022). Nonadherence in Hypertension: How to Develop and Implement Chemical Adherence Testing. Hypertension, 79 (1), 12–23. doi: https://doi.org/10.1161/hypertensionaha.121.17596
- Tan, F. C. J. H., Oka, P., Dambha-Miller, H., Tan, N. C. (2021). The association between self-efficacy and self-care in essential hypertension: a systematic review. BMC Family Practice, 22 (1). doi: https://doi.org/10.1186/s12875-021-01391-2
- Mali, A. D., Bathe, R., Patil, M. (2015). An updated review on transdermal drug delivery systems. International Journal of Advances in Scientific Research, 1 (6), 244–254. doi: https://doi.org/10.7439/ijasr.v1i6.2243
- Pastore, M. N., Kalia, Y. N., Horstmann, M., Roberts, M. S. (2015). Transdermal patches: history, development and pharmacology. British Journal of Pharmacology, 172 (9), 2179–2209. doi: https://doi.org/10.1111/bph.13059
- Singh, I., Morris, A. (2011). Performance of transdermal therapeutic systems: Effects of biological factors. International Journal of Pharmaceutical Investigation, 1 (1), 4–9. doi: https://doi.org/10.4103/2230-973x.76721
- Jeong, W. Y., Kwon, M., Choi, H. E., Kim, K. S. (2021). Recent advances in transdermal drug delivery systems: a review. Biomaterials Research, 25 (1). doi: https://doi.org/10.1186/s40824-021-00226-6
- Vons, B. V., Chubka, M. B., Hroshovyi, T. A. (2017). Transdermal drug delivery system. Pharmaceutical Review, 2, 106–112. doi: https://doi.org/10.11603/2312-0967.2017.2.7902
- Pavan, K. Y., Saurabh M. (2017). Transdermal patch of an antihypertensive drug: its development and evaluation. World Journal of Pharmaceutical Research, 6 (4), 1355–1374. doi: https://doi.org/10.20959/wjpr20174-8214
- Rastogi, V., Pragya, U. P. (2012). A Brief View on Antihypertensive Drugs Delivery through Transdermal Patches. International Journal of Pharmaceutical Sciences and Research, 3 (7), 1955–1970. Available at: https://www.academia.edu/35565081/
- Al Hanbali, O. A., Khan, H. M. S., Sarfraz, M., Arafat, M., Ijaz, S., Hameed, A. (2019). Transdermal patches: Design and current approaches to painless drug delivery. Acta Pharmaceutica, 69 (2), 197–215. doi: https://doi.org/10.2478/acph-2019-0016
- Sharma, P. K., Panda, A., Pradhan, A., Zhang, J., Thakkar, R., Whang, C.-H. et al. (2017). Solid-State Stability Issues of Drugs in Transdermal Patch Formulations. AAPS PharmSciTech, 19 (1), 27–35. doi: https://doi.org/10.1208/s12249-017-0865-3
- Liu, C., Quan, P., Fang, L. (2016). Effect of drug physicochemical properties on drug release and their relationship with drug skin permeation behaviors in hydroxyl pressure sensitive adhesive. European Journal of Pharmaceutical Sciences, 93, 437–446. doi: https://doi.org/10.1016/j.ejps.2016.08.048
- Chandrashekar, N., Shobha Rani, R. (2008). Physicochemical and pharmacokinetic parameters in drug selection and loading for transdermal drug delivery. Indian Journal of Pharmaceutical Sciences, 70 (1), 94–96. doi: https://doi.org/10.4103/0250-474x.40340
- Kokate, A., Li, X., Jasti, B. (2008). Effect of Drug Lipophilicity and Ionization on Permeability Across the Buccal Mucosa: A Technical Note. AAPS PharmSciTech, 9 (2), 501–504. doi: https://doi.org/10.1208/s12249-008-9071-7
- Rahbari, R., Ichim, I., Bamsey, R., Burridge, J., Guy, O. J., Bolodeoku, J., Graz, M. (2020). Characterisation of Drug Delivery Efficacy Using Microstructure-Assisted Application of a Range of APIs. Pharmaceutics, 12(12), 1213. doi: https://doi.org/10.3390/pharmaceutics12121213
- M Elhaj, B., Hamad Farah, F., Saad Ali, H. (2021). Captopril: An Overview of Discovery, Development, and Post-marketingSurveillance as an Effective Anti-hypertensive Drug. Acta Scientific Pharmaceutical Sciences, 5 (4), 6–9. doi: https://doi.org/10.31080/asps.2021.05.0695
- Regulski, Mil. osz, Regulska, K., Stanisz, B., Murias, M., Gieremek, P., Wzgarda, A., Niz.nik, Bartl. omiej. (2015). Chemistry and Pharmacology of Angiotensin-Converting Enzyme Inhibitors. Current Pharmaceutical Design, 21 (13), 1764–1775. doi: https://doi.org/10.2174/1381612820666141112160013
- Patel, R. N. (2016). Applications of Biocatalysis for Pharmaceuticals and Chemicals. Organic Synthesis Using Biocatalysis. Academic Press, 339–411. doi: https://doi.org/10.1016/b978-0-12-411518-7.00011-1
- Panchyshyn, Yu. M. (2012). Praktychni aspekty zastosuvannia inhibitoriv APF u khvorykh iz kardiovaskuliarnoiu patolohiieiu. Ratsionalna farmakoterapiia, 3 (24), 22–29. Available at: https://rpht.com.ua/ua/archive/2012/3%2824%29/pages-22-29/praktichni-aspekti-zastosuvannya-ingibitoriv-apf-u-hvorih-iz-kardiovaskulyarnoyu-patologiieyu
- Piepho, R. W. (2000). Overview of the angiotensin-converting-enzyme inhibitors. American Journal of Health-System Pharmacy, 57 (1), S3–S7. doi: https://doi.org/10.1093/ajhp/57.suppl_1.s3
- Derzhavna Farmakopeia Ukrainy (2015). Kharkiv: Derzhavne pidpryiemstvo «Ukrainskyi naukovyi- farmakopeinyi tsentr yakosti likarskykh zasobiv», 1128.
- European Pharmacopoeia. European Directorate for the Quality of Medicines (2019). Strasbourg, 4370.
- Andújar-Sánchez, M., Cámara-Artigas, A., Jara-Pérez, V. (2004). A calorimetric study of the binding of lisinopril, enalaprilat and captopril to angiotensin-converting enzyme. Biophysical Chemistry, 111 (2), 183–189. doi: https://doi.org/10.1016/j.bpc.2004.05.011
- Hillaert, S., Van den Bossche, W. (2000). Optimization of capillary electrophoretic separation of several inhibitors of the angiotensin-converting enzyme. Journal of Chromatography A, 895 (1-2), 33–42. doi: https://doi.org/10.1016/s0021-9673(00)00591-4
- Remko, M. (2006). Acidity, Lipophilicity, Solubility, Absorption, and Polar Surface Area of Some ACE Inhibitors. Chemical Papers, 61 (2), 133–141. doi: https://doi.org/10.2478/s11696-007-0010-y
- Spiller, H. A. (2014). Angiotensin Converting Enzyme (ACE) Inhibitors. Encyclopedia of Toxicology, 14–16. doi: https://doi.org/10.1016/b978-0-12-386454-3.00686-2
- Foye, W. O., Lemke, T. L., Williams, D. A. (2008). Foye's principles of medicinal chemistry. Lippincott Williams & Wilkins. Available at: https://www.worldcat.org/title/foyes-principles-of-medicinal-chemistry/oclc/145942325
- Di, L., Kerns, E. H. (2003). Profiling drug-like properties in discovery research. Current Opinion in Chemical Biology, 7 (3), 402–408. doi: https://doi.org/10.1016/s1367-5931(03)00055-3
- Hartmann, T., Schmitt, J. (2004). Lipophilicity – beyond octanol/water: a short comparison of modern technologies. Drug Discovery Today: Technologies, 1 (4), 431–439. doi: https://doi.org/10.1016/j.ddtec.2004.10.006
- Odovic, J., Markovic, B., Trbojevic-Stankovic, J., Vladimirov, S., Karljikovic-Rajic, K. (2013). Evaluation of ACE inhibitors lipophilicity using in silico and chromatographically obtained hydrophobicity parameters. Hemijska Industrija, 67 (2), 209–216. doi: https://doi.org/10.2298/hemind120522078o
- Bruno, C. D., Harmatz, J. S., Duan, S. X., Zhang, Q., Chow, C. R., Greenblatt, D. J. (2021). Effect of lipophilicity on drug distribution and elimination: Influence of obesity. British Journal of Clinical Pharmacology, 87 (8), 3197–3205. doi: https://doi.org/10.1111/bcp.14735
- Remko, M., Swart, M., Bickelhaupt, F. M. (2006). Theoretical study of structure, pKa, lipophilicity, solubility, absorption, and polar surface area of some centrally acting antihypertensives. Bioorganic & Medicinal Chemistry, 14 (6), 1715–1728. doi: https://doi.org/10.1016/j.bmc.2005.10.020
- Stella, V. J., Borchardt, R. T., Hageman, M. J., Oliyai, R., Maag, H., Tilley, J. W. (Eds.) (2007). Prodrugs: Challenges and Rewards. Biotechnology: Pharmaceutical Aspects. New York: Springer Science, Business Media, 1464. doi: https://doi.org/10.1007/978-0-387-49785-3
- Trbojevic, J., Odovic, J., Trbojevic-Stankovic, J., Stojimirovic, B., Jelic, R. (2017). The Evaluation of Angiotensin-Converting Enzyme Inhibitors in Renal Elimination with Selected Molecular Descriptors. Serbian Journal of Experimental and Clinical Research, 18 (2), 119–123. doi: https://doi.org/10.1515/sjecr-2016-0100
- Barna, O. N. (2015). Mistse Dyrotonu sered inhibitoriv APF: vid farmakokinetyky do farmakoekonomiky. Zdorov’ia Ukrainy. Medychni vydannia. Available at: https://health-ua.com/article/18708-mstce-dirotonu-sered-ngbtorv-apf-vd-farmakoknetiki-do-farmakoekonomki
- Zheng, W., Tian, E., Liu, Z., Zhou, C., Yang, P., Tian, K. et al. (2022). Small molecule angiotensin converting enzyme inhibitors: A medicinal chemistry perspective. Frontiers in Pharmacology, 13. doi: https://doi.org/10.3389/fphar.2022.968104
- Gullick, D. R., Pugh, W. J., Ingram, M. J., Cox, P. A., Moss, G. P. (2010). Formulation and characterization of a captopril ethyl ester drug-in-adhesive-type patch for percutaneous absorption. Drug Development and Industrial Pharmacy, 36 (8), 926–932. doi: https://doi.org/10.3109/03639040903585135
- Duraivel, S., Rajalakshmi, A.N., Debjit B. (2014). Formulation and evaluation of captopril Transdermal Patches. Elixir Pharmacy, 76, 28209–28213.
- Pravin, U., Akshata, K., Pravin, C., Manoj, D., Vishal, D. (2016). Formulation and Evaluation of Captopril Transdermal patches for the treatment of hypertension. Available online at www.scholarsresearchlibrary.com. Scholars Research Library. Der Pharmacia Lettre, 8 (5), 12–16.
- Dhanabal, C. (2019). Formulation and Evaluation of Transdermal Delivery System Comprising Captopril as Antihypertensive Drug. Coimbatore: RVS College of Pharmaceutical Sciences.
- Kerımoğlu, O., Şahbaz, S., Şehırlı, Ö., Ozdemır, Z. N., Şule Çetınel, Dortunç, B., Şener, G. (2015). Pharmacodynamical evaluation of matrix type transdermal therapeutic systems containing captopril. Acta Pol Pharm., 72 (4), 799–806. Available at: https://pubmed.ncbi.nlm.nih.gov/26647638/
- Natrajan, R., Rajendran, N.N., Sabareesh, M., Selvaraj, S. (2011). Formulation and evaluation of transdermal delivery of enalapril maleate. International Journal of Pharmaceutical Research., 3, 73–78. Available at: https://www.researchgate.net/publication/287472077_Formulation_and_evaluation_of_transdermal_delivery_of_enalapril_maleate
- Gavali, P., Gaikwad, A., Radhika, P. R., Sivakumar, T. (2010). Design and development of hydroxypropyl methylcellulose (HPMC) based polymeric film of enalapril maleate. InternationalJournal of PharmTech Research, 2 (1), 274–282. Available at: https://sphinxsai.com/sphinxsaivol_2no.1/pharmtech_vol_2no.1/PharmTech_Vol_2No.1PDF/PT%20=44%20(274-282).pdf
- Aqil, M., Bhavna, Chowdhary, I., Sultana, Y., Talegaonkar, S., Ahmad, F. J., Ali, M. M. (2008). Transdermal Therapeutic System of Enalapril Maleate Using Piperidine as Penetration Enhancer. Current Drug Delivery, 5 (2), 148–152. doi: https://doi.org/10.2174/156720108783954860
- Pramod, K. P., Ramesh, K., Rashmi, M. B., Rajarajan, S. (2019). Formulation and Evaluation of Transdermal Patches of an Antihypertensive Drug for Controlled Release. Journal of Emerging Technologies and Innovative Research (JETIR), 6 (6), 444–452. Available at: https://www.jetir.org/papers/JETIR1906210.pdf
- Sabareesh, M., Yanadaiah, J. P., Chandra Sekhar, K. B. (2020). A Novel Vesicular Approach For Transdermal Administration Of Enalapril Maleate Loaded Nanoproniosomal Gel: Formulation, Ex Vivo Evaluation And In Vivo Antihypertensive Study. International Journal of Applied Pharmaceutics, 12 (5), 190–202. doi: https://doi.org/10.22159/ijap.2020v12i5.38463
- Jitendra, B., Pathaka, A. K., Subhash. P. (2010). Development and Optimization of Transdermal System of Lisinopril dehydrate: Employing Permeation Enhancers. Iranian Journal of Pharmaceutical Sciences, 6 (4). Available at: http://www.ijps.ir/article_2166.html
- Rajendra, M., Srinivas, A. (2015). Formulation and Characterization Transdermal Patches for Enhancing the Bio-Availability of Lisinopril Dihydrate. International Journal of Medicine and Nanotechnology, 2 (6), 306–310. Available at: https://www.researchgate.net/publication/296573307
- Aparanjitha, R., Sunitha, R. M., Sarangapani, M. (2020). Formulation And In Vitro Evaluation of Transferosomal Patches for Enhanced Drug Delivery of Lisinopril Dihydrate. Journal of Scientific Research, 64 (3), 105–109. doi: https://doi.org/10.37398/jsr.2020.640318
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Tatyana Shyteyeva, Elena Bezchasnyuk, Oleg Kryskiv, Vasyl Grynenko

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






