Study of factors affecting some properties of hydrophilic suppository base
DOI:
https://doi.org/10.15587/2519-4852.2023.286315Keywords:
suppository, base, solvent, excipient, resistance to rupture, disintegration time, release, water absorptionAbstract
The aim. To study the effect of the composition of hydrophilic suppository bases on the physicochemical and osmotic properties of suppositories made from them.
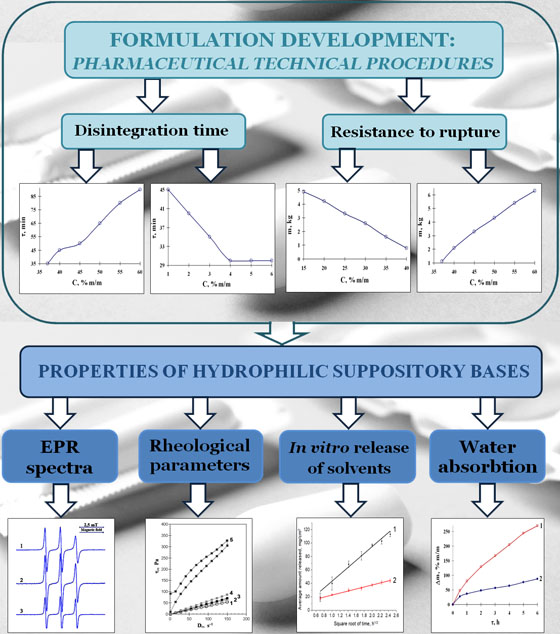
Materials and methods. The bases were studied with varying compositions of excipients. The microstructure of the bases was evaluated, and the disintegration time and resistance to rupture of suppositories made from them were determined. The kinetics of water absorption and solvent release were studied by dialysis. The content of propylene glycol (PG) and macrogol 400 (M400) in the receptor medium was determined by gas chromatography. The melted bases were studied by rotational viscometry. The electron paramagnetic resonance spectra of spin probes in hydrophilic solvents and bases were obtained; the type of spectrum, isotropic constant (AN), rotational correlation times (τ), and anisotropy parameter (ε) were determined.
Results. The disintegration times and resistance to rupture of suppositories were determined depending on such factors as the content and grade of poloxamers, the ratio between high molecular weight macrogols and the mixed solvent PG-M400 (60 : 40 % m/m), the ratio of nonionic surfactant and cetostearyl alcohol (CSA) and their total content, water and hard fat content. The introduction of solid fat and a mixture of surfactants and CSA provides the uniform structure of the bases. The mass ratio between surfactants and CSA and their total content are important factors that provide acceptable resistance to rupture and disintegration times for suppositories and reduce water absorption and solvent release. As the temperature decreases from 45 °C to 20 °C, the bases transform from Newtonian liquids to solids. At that time, the microviscosity of the environment of the spin probes increased by about 5 times, but the parameters of their rotational diffusion in solid bases and the mixed solvent PG-M400 are comparable. This indicates the dissolved state of the spin probes in the bases and the absence of the formation of mixed associates from molecules of surfactant and CSA.
Conclusions. By varying the composition of excipients, the properties of hydrophilic suppository bases can be controlled, significantly reducing their osmotic properties. The active substances in these bases may be in a dissolved state due to the high content of non-aqueous solvents
References
- Buckingham, R. (Ed.) (2020). Martindale: The Complete Drug Reference. London: Pharmaceutical Press, 4912.
- Ul Haq, M. M., Ur Razzak, M. M., Uddin, M. A., Ahmed, N., Shahidulla, S. M. (2021). Rectal Drug Delivery System: An Overview. Clinical Pharmacology and Biopharmaceutics, 10 (5), 219.
- Hua, S. (2019). Physiological and Pharmaceutical Considerations for Rectal Drug Formulations. Frontiers in Pharmacology, 10. doi: https://doi.org/10.3389/fphar.2019.01196
- Melnyk, G., Yarnykh, T., Herasymova, I. (2020). Analytical Review of the Modern Range of Suppository Bases. Systematic Reviews in Pharmacy, 11 (4), 503–508. doi: https://doi.org/10.31838/srp.2020.4.76
- Sheskey, P. J., Hancock, B. C., Moss, G. P., Goldfarb, D. J. (Ed.) (2020). Handbook of Pharmaceutical Excipients. London: Pharm. Press, 1296.
- Szulc-Musiol, B., Bulas, L., Dolinska, B. (2019). Effect of Selected Surfactants on Kinetics of Meloxicam Release from Rectal Suppositories. Indian Journal of Pharmaceutical Sciences, 81 (6), 1115–1121. doi: https://doi.org/10.36468/pharmaceutical-sciences.610
- Ilomuanya, M. O., Ifudu, Ndu. D., Odulaja, J., Igwilo, C. (2012). Assessment of the effect of base type and surfactant on the release properties and kinetics of paracetamol suppositories. Journal of Chemical and Pharmaceutical Research, 4 (6), 3280–3286.
- Kasperek, R., Galczynski, K., Nalesniak, M., Iwaniak, K., Poleszak, E. (2014). Influence of the dissolution medium on the release of dehydroepiandrosterone from lipophilic suppositories. Current Issues in Pharmacy and Medical Sciences, 27 (1), 46–50. doi: https://doi.org/10.2478/cipms-2014-0012
- The European Pharmacopoeia (2022). EDQM. Strasbourg: Council of Europe. Available at: http://pheur.edqm.eu/subhome/11-0
- Hanning, S. M., Matiz, S., Krasser, K., Orlu, M., Dodoo, C., Gaisford, S., Tuleu, C. (2020). Characterisation of rectal amoxicillin (RAMOX) for the treatment of pneumonia in children. Drug Delivery and Translational Research, 11 (3), 944–955. doi: https://doi.org/10.1007/s13346-020-00804-6
- Akhtar, M., Akhtar, N., Ahmad, M., Arif, A. S., Shah, P. A. (2000). Drug release from PEG suppository bases and from their combination with polymers. Journal of Faculty of Pharmacy of Istanbul University, 33, 7–16.
- Zawar, L. R., Bhandari, G. S. (2012). Formulation and Evaluation of Sustained Release Ondansetron Poloxamer Based Solid Suppositories. Journal of Applied Pharmaceutical Science, 2 (7), 186–190. doi: https://doi.org/10.7324/japs.2012.2729
- Havaldar, V. D., Yadav, A. V., Dias, R. J., Mali, K. K., Survase, A. B., Ghorpade, V. S., Salunkhe, N. H. (2017). Screening of Suppository bases for Rectal delivery of Carbamazepine. Research Journal of Pharmacy and Technology, 10 (8), 2697–2703. doi: https://doi.org/10.5958/0974-360x.2017.00479.6
- Ali, M. M., Mashrai A., Al-dholimi, N. (2018). Sustained Release Suppositories of Metoclopramide HCl: Formulation and In vitro Evaluation. Journal of Chemical and Pharmaceutical Research, 10 (1), 169–175.
- Mori, K., Hori, S., Kawata, T., Kogure, S., Matsumoto, K., Hasegawa, T., Akimoto, M. (2017). The in Vitro Release of Indomethacin from Suppositories: Effects of Bases and Comparison of Different Dissolution Methods. Chemical and Pharmaceutical Bulletin, 65 (7), 674–677. doi: https://doi.org/10.1248/cpb.c16-00794
- El Majri, M. A., El Baseir, M. M. (2016). Formulation and evaluation of ibuprofen suppositories. International Research Journal of Pharmacy, 7 (6), 87–90. doi: https://doi.org/10.7897/2230-8407.07670
- Bialik, M., Kuras, M., Sobczak, M., Oledzka, E. (2021). Achievements in Thermosensitive Gelling Systems for Rectal Administration. International Journal of Molecular Sciences, 22 (11), 5500. doi: https://doi.org/10.3390/ijms22115500
- Ban, E., Kim, C.-K. (2013). Design and evaluation of ondansetron liquid suppository for the treatment of emesis. Archives of Pharmacal Research, 36 (5), 586–592. doi: https://doi.org/10.1007/s12272-013-0049-y
- Bodratti, A., Alexandridis, P. (2018). Formulation of Poloxamers for Drug Delivery. Journal of Functional Biomaterials, 9 (1), 11. doi: https://doi.org/10.3390/jfb9010011
- Liapunov, N. A., Bezuglaia, E. P., Fadeikina, A. G., Lysokobylka, A. A., Stolper, Iu. M. (1999). Sozdanie miagkikh lekarstvennykh sredstv na razlichnykh osnovakh. Soobshchenie 1. Issledovanie reologicheskikh svoistv mazei na vodorastvorimykh osnovakh. Farmakom, 6, 10–16.
- Bezugla, O. P., Lyapunov, M. O., Zinchenko, I. O., Lisokobilka, O. A., Liapunova, A. M. (2022). Modeling of processes of solvent diffusion from ointment bases using in vitro experiments. Functional materials, 29 (4), 553–558. doi: https://doi.org/10.15407/fm29.04.553
- Lyapunov, N., Bezuglaya, E., Liapunova, A., Zinchenko, I., Liapunov, O., Lysokobylka, O., Stolper, Y. (2022). Effect of the composition of emulsifiers and the dispersion medium on the properties of bases for semi-solid preparations. ScienceRise: Pharmaceutical Science, 5 (39), 29–45. doi: https://doi.org/10.15587/2519-4852.2022.266001
- Bezuglaya, E., Krasnopyorova, A., Liapunova, A., Zinchenko, I., Lyapunov, N., Sytnik, O. (2023). Influence of physicochemical properties and structure of mixed solvents propylene glycol – macrogol 400 on their in vitro release. ScienceRise: Pharmaceutical Science, 1 (41), 4–13. doi: https://doi.org/10.15587/2519-4852.2023.274468
- Derzhavna Farmakopeia Ukrainy. Druhe vydannia. Vol. 1 (2015). Kharkiv: Derzhavne pidpryiemstvo «Ukrainskyi naukovyi farmakopeinyi tsentr yakosti likarskykh zasobiv», 1128.
- Derzhavna Farmakopeia Ukrainy. Pershe vydannia. Dopovnennia 1 (2004). Kharkiv: Derzhavne pidpryiemstvo «Naukovo-ekspertnyi farmakopeinyi tsentr», 520.
- Draft guideline on quality and equivalence of topical products (2018). CHMP/QWP/708282/2018. Available at: www.ema.europa.eu/en/quality-equivalence-topical-products
- The United States Pharmacopoeia, 41 – NF 36 (2018). The United States Pharmacopoeial Convention. Rockville. Available at: https://www.worldcat.org/title/united-states-pharmacopeia-2018-usp-41-the-national-formulary-nf-36/oclc/1013752699
- Ilić, T., Pantelić, I., Savić, S. (2021). The Implications of Regulatory Framework for Topical Semisolid Drug Products: From Critical Quality and Performance Attributes towards Establishing Bioequivalence. Pharmaceutics, 13 (5), 710. doi: https://doi.org/10.3390/pharmaceutics13050710
- Tiffner, K. I., Kanfer, I., Augustin, T., Raml, R., Raney, S. G., Sinner, F. (2018). A comprehensive approach to qualify and validate the essential parameters of an in vitro release test (IVRT) method for acyclovir cream, 5%. International Journal of Pharmaceutics, 535 (1-2), 217–227. doi: https://doi.org/10.1016/j.ijpharm.2017.09.049
- Bezuglaya, E., Lyapunov, N., Bovtenko, V., Zinchenko, I., Stolper, Y. (2021). Study of pressurised metered dose inhalers for the purpose of standardization of quality attributes characterizing uniformity of dosing. ScienceRise: Pharmaceutical Science, 4 (32), 11–23. doi: https://doi.org/10.15587/2519-4852.2021.238294
- Kuznetcov, A. N. (1976). Metod spinovogo zonda (Osnovy i primenenie). Moscow: Nauka, 210.
- Likhtenshtein, G. I. (1974). Metod spinovykh zondov v molekuliarnoi biologii. Moscow: Nauka, 256.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Elena Bezuglaya, Yurij Stolper, Nikolay Lyapunov, Igor Zinchenko, Oleksii Liapunov

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






