Pharmaceutical providing of the treatment of children with epilepsy in Ukraine and abroad
DOI:
https://doi.org/10.15587/2519-4852.2023.286425Keywords:
epilepsy, antiepileptic drugs, pharmaceutical providing, clinical protocols, registration, economic availability, national pharmaceutical market, marketing analysisAbstract
The aim: to investigate the pharmaceutical providing for the treatment of epilepsy in children in Ukraine and abroad and to conduct a marketing analysis of the domestic market of antiepileptic drugs.
Materials and methods: Ukrainian and foreign Internet sources, medical and technological documents on a specific research topic, the regulatory and legal base of Ukraine, materials of the State Formulary of Medicines, the State Register of Medicines of Ukraine were the objects of the study. The methods of marketing analysis, graphic, documentary and analytical generalization were used.
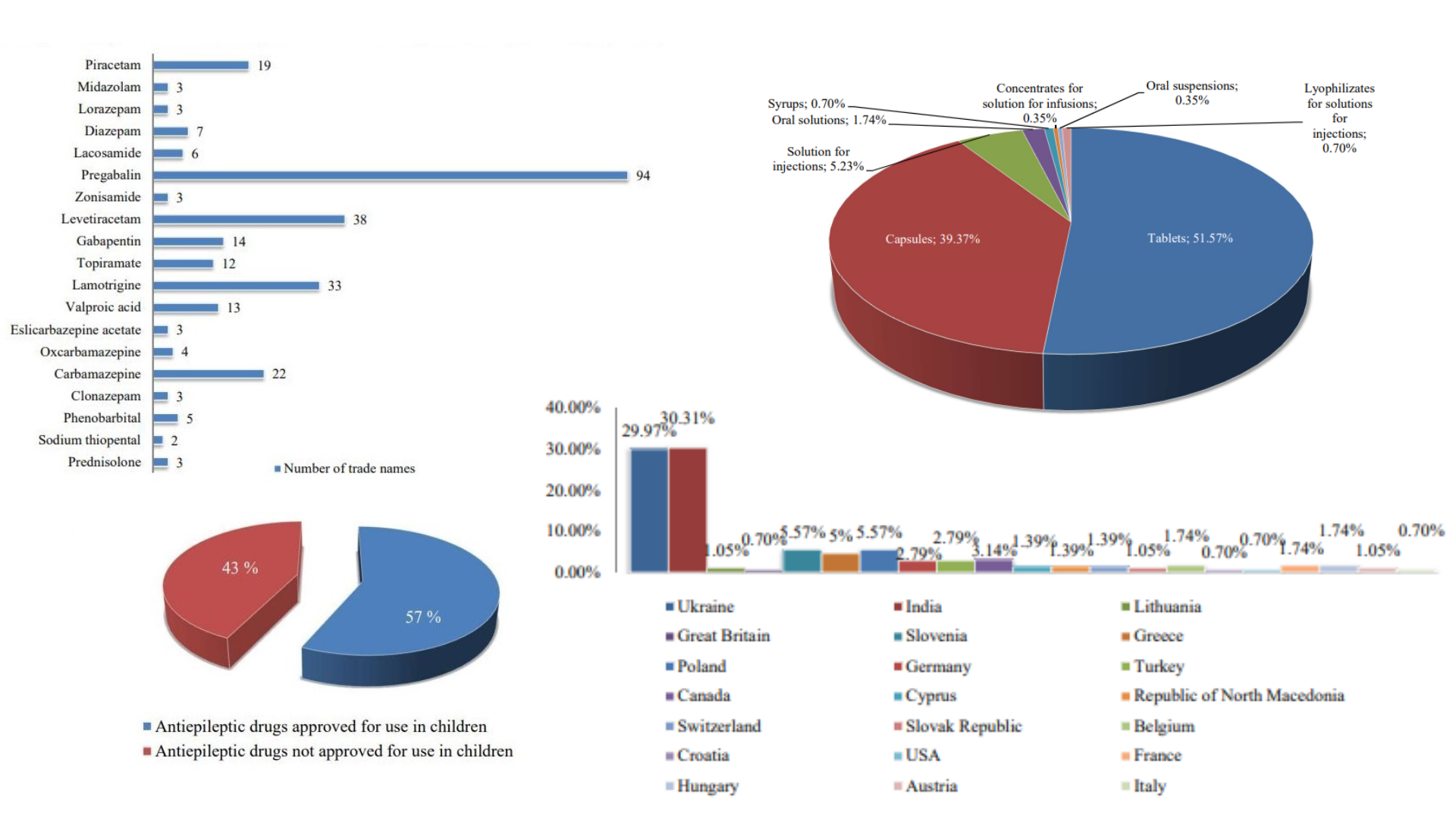
Results: a comparative analysis of clinical protocols (France, Great Britain and Ukraine) for the treatment of epilepsy in children showed that there are only 12 INNs out of 37 INNs in the country. The analysis of the formulary lists of drugs of Great Britain and Ukraine showed that the BNFC has 32 INNs, and the domestic - 10 INNs. As of October 2022, 287 names of antiepileptic drugs are registered in Ukraine, and 164 of them are allowed in children's practice. The main producers of antiepileptic drugs are India and Ukraine. Children's drugs forms are mainly represented by tablets and capsules - more than 90%. A comparison of the lists of drugs for the treatment of epilepsy from the National List of Essential Medicines of Ukraine, BNFC, WHO Model List of Essential Medicines and WHO Model List of Essential Medicines for Children showed that out of 37 INNs, only 11 INNs are listed in all documents.
Conclusions: the results prove the need of improvement of pharmaceutical providing of epilepsy treatment in Ukraine. The nomenclature and variety of dosage forms for children's practice need to be expanded. In order to increase the economic availability of epilepsy treatment, it is advisable to update the National List of Essential Medicines of Ukraine and include new drugs in it, which will make it possible to purchase them at the expense of the State Budget of Ukraine
References
- Epilepsy (2023). Available at: https://www.who.int/news-room/fact-sheets/detail/epilepsy
- Panayiotopoulos C.P. (2010). Epileptic Syndromes and their Treatment. London: Springer¬Verag, 620.
- Shcho treba znaty pro epilepsiiu (2019). Available at: https://www.phc.org.ua/news/scho-treba-znati-pro-epilepsiyu
- Polozhennia pro unifikovanyi klinichnyi protokol pervynnoi, ekstrenoi, vtorynnoi (spetsializovanoi) ta tretynnoi (vysokospetsializovanoi) medychnoi dopomohy "Epilepsii u ditei" (2014). Nakaz MOZ Ukrainy No. 276 v0276282-14. 17.04.2014. Available at: zakon.rada.gov.ua/rada/show/v0276282-14#Text
- Kozolkin, O. A., Vizir, I. V., Sikorska, M. V. (2019). Epilepsiia. Suchasni pryntsypy diahnostyky i likuvannia. Zaporizhzhia: ZDMU, 153. Available at: http://dspace.zsmu.edu.ua/handle/123456789/12135
- Who We Are. European Academy of Neurology. Available at: https://www.ean.org/home/about-us/who-we-are
- About the American Neurological Association (2022). American Neurological Association. Available at: https://myana.org/about-american-neurological-association
- British Paediatric Neurology Association. Available at: bpna.org.uk/
- Epilepsy Foundation. Available at: epilepsy.com/about-us/about-foundation
- Association Epilepsie-France. Available at: http://www.epilepsie-france.com/notre-association/qui-sommes-nous.html
- International League Against Epilepsy. Available at: https://www.ilae.org/
- Épilepsies: Prise en charge des enfants et des adultes. Recommandation. Available at: has-sante.fr/upload/docs/application/pdf/2020-10/reco308_recommandations_epilepsies_preparation_mel.pdf
- Epilepsies in children, young people and adults. NICE guideline [NG217] (2022). Available at: https://www.nice.org.uk/guidance/NG217
- British National Formulary for Children 2019–2020. Available at: https://nhathuocngocanh.com/wp-content/uploads/pdf/BNFC-2019-2020.pdf
- Unifikovanyi klinichnyi protokol pervynnoi, ekstrenoi, vtorynnoi (spetsializovanoi) ta tretynnoi (vysokospetsializovanoi) medychnoi dopomohy "Epilepsii u ditei". Available at: https://www.dec.gov.ua/wp-content/uploads/2019/11/2014_276_ykpmd_epilepsiya_dity.pdf
- Pro zatverdzhennia chotyrnadtsiatoho vypusku Derzhavnoho formuliara likarskykh zasobiv ta zabezpechennia yoho dostupnosti (2022). Nakaz MOZ Ukrainy No. 1011. 13.06.2022. Available at: https://moz.gov.ua/article/ministry-mandates/nakaz-moz-ukraini-vid-13062022--1011-pro-zatverdzhennja-chotirnadcjatogo-vipusku-derzhavnogo-formuljara-likarskih-zasobiv-ta-zabezpechennja-jogo-dostupnosti
- Epidyolex (cannabidiol). An overview of Epidyolex and why it is authorised in the EU. EMA/191061/2021. Available at: https://www.ema.europa.eu/en/documents/overview/epidyolex-epar-medicine-overview_en.pdf
- Burns, C. (2019). NICE recommends first cannabis-based medicines for use on the NHS. The Pharmaceutical Journal, 303 (7931). doi: https://doi.org/10.1211/pj.2019.20207320
- Aliekperova, N., Kosyachenko, K., Kaniura, O. (2020). Perspectives on formation of medical cannabis market in Ukraine based on holistic approach. Journal of Cannabis Research, 2 (1). doi: https://doi.org/10.1186/s42238-020-00044-y
- Alekperova, N. V. (2019). Analysis of drugs that are used in the cannabis market for medical purposes. Retsept, 22 (6), 938–945.
- Sholoiko, N. V., Popov, V. I., Lysenko, T. I. (2019). Suchasnyi stan ta perspektyvy zastosuvannia konopel u medytsyni ta farmatsii (Ohliad literatury). Fitoterapiia. Chasopys, 1, 44–52.
- Pro vnesennia zmin do nakazu Ministerstva okhorony zdorov’ia Ukrainy vid 28 veresnia 2012 roku № 751 (2016). Nakaz MOZ Ukrainy No. 1422 z0530-17. 29.12.2016. Available at: https://zakon.rada.gov.ua/laws/show/z0530-17#Text
- Polozhennia pro Derzhavnyi reiestr likarskykh zasobiv (2004). Postanova Kabinetu Ministriv Ukrainy No. 411. 31.03.2004. Available at: https://zakon.rada.gov.ua/laws/show/411-2004-%D0%BF#Text
- "Derzhavnyi reiestr likarskykh zasobiv Ukrainy" Informatsiinyi fond. Available at: http://www.drlz.com.ua/
- Perucca, E. (2002). Pharmakological and therapeutic properties of valproate. A summary after 35 year of clinical experience. CNS Drugs, 16 (10), 695–714. doi: https://doi.org/10.2165/00023210-200216100-00004
- Hirsch, L. J., Weintraub, D. B., Buchsbaum, R., Spencer, H. T., Straka, T., Hager, M., Resor, S. R. (2006). Predictors of Lamotrigine-associated Rash. Epilepsia, 47 (2), 318–322. doi: https://doi.org/10.1111/j.1528-1167.2006.00423.x
- Teresk, M. G., Berkland, C. J., Dormer, N. H. (2017). Deficiencies in Traditional Oral Dosage Forms and the Emergence of Controlled-Release Powder Manufacturing. KONA Powder and Particle Journal, 34, 91–105. doi: https://doi.org/10.14356/kona.2017013
- Kale, R. (2002). The Treatment Gap. Epilepsia, 43, 31–33. doi: https://doi.org/10.1046/j.1528-1157.43.s.6.13.x
- Horachuk, V. V. (2011). Medical, social and economic aspects of epilepsy. Ukrainskyi medychnyi chasopys, 5 (85), 9–10. Available at: https://www.umj.com.ua/article/18382/mediko-socialni-ta-ekonomichni-aspekti-epilepsii
- Vitchyzniana epileptolohiia: profesiini dosiahnennia ta mizhnarodne vyznannia (2021). NeiroNews: psykhonevrolohiia ta neiropsykhiatriia, 1 (122). Available at: https://neuronews.com.ua/ua/archive/2021/1%28122%29/pages-11-15/vitchiznyana-epileptologiya-profesiyni-dosyagnennya-ta-mizhnarodne-viznannya#gsc.tab=0
- World Health Organization model list of essential medicines: 21st list 2019 (2019). Geneva, 2019. Available at: https://www.who.int/publications/i/item/WHOMVPEMPIAU2019.06
- Shaw, B. (2019). The WHO’s Essential Medicines List: Changing the Conversation. Available at: https://pharmaboardroom.com/articles/the-whos-essential-medicines-list-changing-the-conversation/
- Pro likarski zasoby (1996). Zakon Ukrainy No. 123/96-VR. 04.04.1996. Available at: https://zakon.rada.gov.ua/laws/show/123/96-%D0%B2%D1%80#Text
- Osnovy zakonodavstva Ukrainy pro okhoronu zdorov’ia (1993). Zakon Ukrainy No. 2801-XII. 19.11.1992. Available at: https://zakon.rada.gov.ua/laws/show/2801-12#Text
- Dostupni liky. Available at: https://moz.gov.ua/dostupni-liki
- Yak pratsiuie prohrama reimbursatsii «Dostupni liky» z onovlenym reiestrom likarskykh zasobiv (2023). Available at: https://medplatforma.com.ua/article/1975-yak-pratsyu-programa-rembursats-dostupn-lki-z-1-jovtnya
- World Health Organization Model List of Essential Medicines for Children (2021). Geneva: World Health Organization. Available at: https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2021.03
- Pro derzhavni finansovi harantii medychnoho obsluhovuvannia naselennia (2017). Zakon Ukrainy No. 2168-VIII. 19.10.2017. Available at: https://zakon.rada.gov.ua/laws/show/2168-19#Text
- Gonchar, A., Sholoiko, N. (2022). Consumption analysis of two-component fixed combinations of medicines for arterial hypertension treatment in Ukraine as one of the stages for evaluation of their reimbursement prospects. ScienceRise: Pharmaceutical Science, 4 (38), 19–27. doi: https://doi.org/10.15587/2519-4852.2022.263733
- Fedotova, M., Panfilova, H., Hala, L., Lebedyn, A., Simonian, L., Gerush, O. et al. (2022). Evaluation of the state of pharmaceutical supply of patients with dementia with Alzheimer disease in Ukraine in accordance with international recommendations. ScienceRise: Pharmaceutical Science, 4 (38), 53–61. doi: https://doi.org/10.15587/2519-4852.2022.263415
- Kostiuk, I. A. (2019). Analysis of dynamics of the medcine list in the government program «available medicines» for treatment of bronchial asthma. Farmatsevtychnyi Zhurnal, 5 (74), 12–20. doi: https://doi.org/10.32352/0367-3057.5.19.02
- Vlasenko, I. O., Davtian, L. L. (2021). Pharmaceutical provision of insulin in Ukraine 2016–2021. Pharmaceutical Review, 2, 55–64. doi: https://doi.org/10.11603/2312-0967.2021.2.12176
- Hrynkiv, Ya. O., Blavatska, O. B., Lozynska, M. O. (2015). Information-organizational providing of pharmaceutical care for patients with epilepsy. Pharmaceutical Review, 2, 68–71. Available at: http://nbuv.gov.ua/UJRN/Phch_2015_2_15
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Natalia Sholoiko, Liliia Hala, Kostyantyn Kosyachenko, Myroslava Hubar

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






