Current approaches of health technologies introduction in Ukrainian hospitals
DOI:
https://doi.org/10.15587/2519-4852.2023.289683Keywords:
hospital, health technology, health technology assessment (HTA), hospital-based health technology assessment (HB-HTA), hospital managers, semi-structured interviewAbstract
The study aimed to gather insights regarding current decision-making approaches and identify challenges and opportunities related to health technology implementation in Ukrainian hospitals.
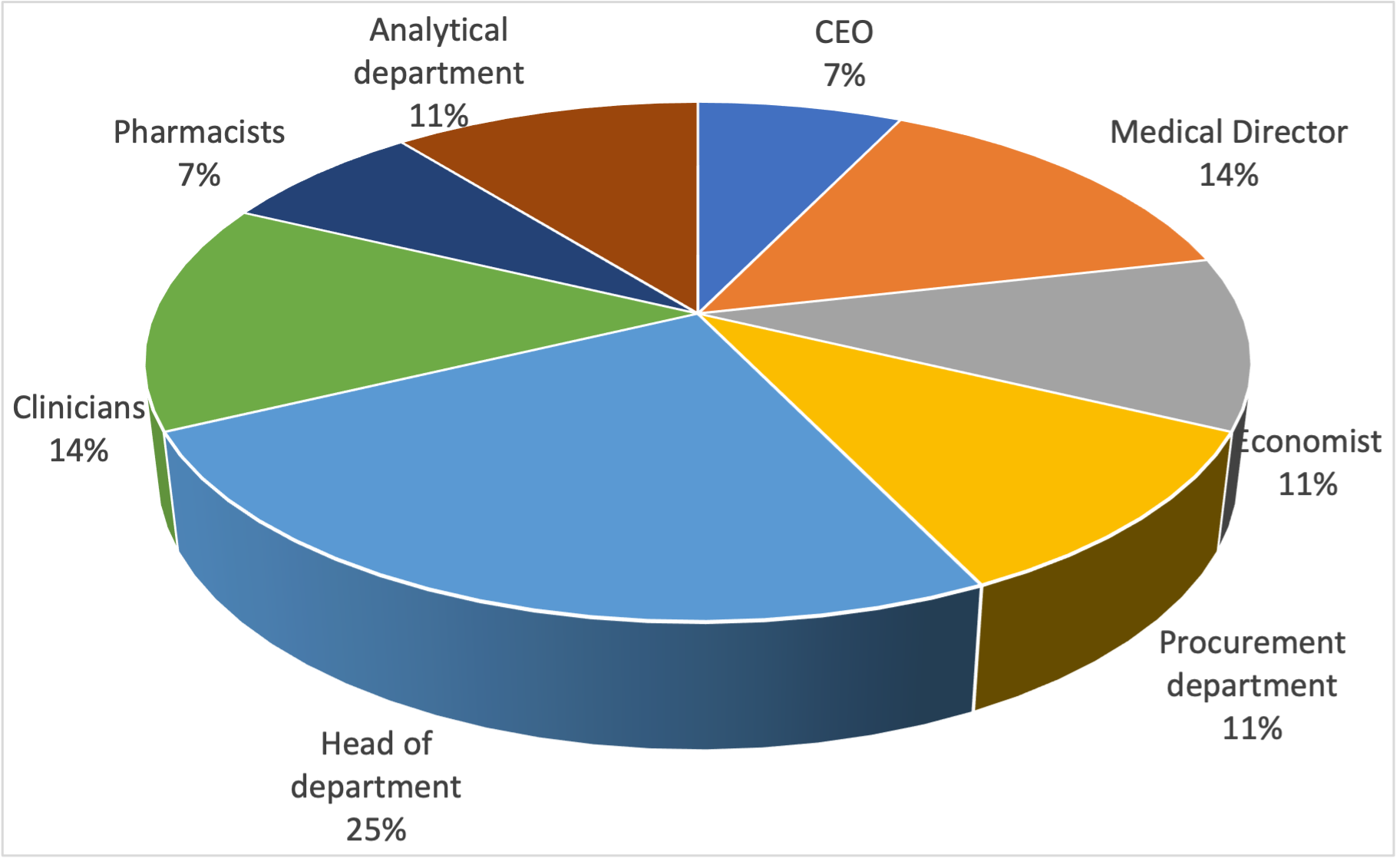
Materials and methods. A total of 28 respondents from three public hospitals across different regions of Ukraine participated in online semi-structured interviews conducted between September 2022 and March 2023. The respondents for this study encompassed a diverse range of stakeholders, including general managers, medical directors, economists, representatives from procurement departments, heads of clinical and diagnostic departments, clinicians, and pharmacists. To effectively guide the interview process, a comprehensive questionnaire was developed.
The data collected from the respondents were analyzed and synthesized to results and conclusions.
Results. The semi-structured interview study consistently revealed a common pattern in all the interviewed hospitals regarding the adoption of new health technologies.
The process of introducing these technologies entails several stages, each demanding meticulous planning, coordination, and active engagement from various stakeholders. What stood out was that stakeholders from the same speciality were consistently involved in the same decision-making stage across all the hospitals we studied.
The technical stages of implementing new health technologies, as outlined by the Law of Ukraine "On Public Procurement," are well-established in Ukrainian hospitals. However, there is room for improvement in selecting which type of health technology to procure. The current assessment process often relies on limited sources of information, lacking comprehensive literature reviews or robust comparisons with existing practices or placebos. This information gap hampers the ability to make well-informed decisions that account for all relevant factors.
The study also identifies specific challenges for the future implementation of the Hospital Health Thechnology Assessment (HB-HTA) in Ukraine. These challenges necessitate investment in building HTA expertise at the hospital level, cultivating strong leadership support, providing professional staff training, and establishing robust data collection and management systems. Overcoming these obstacles is crucial for hospitals to bolster their capacity to effectively implement new health technologies and make informed decisions about their utilization.
Conclusions. Our study has revealed that all the technical stages involved in implementing new health technologies in Ukraine are well established. It was observed that there is room for improvement in the process of selecting which new health technology to purchase. This stage, known as the decision-making process based on HTA, requires implementation at the hospital level.
Our study highlights the evident interest and potential benefits associated with adopting HB-HTA in the hospitals under investigation. By integrating HB-HTA into existing practices and approaches, hospitals can significantly enhance their decision-making processes when introducing new health technologies.
There is an urgent need to introduce the term "hospital-based HTA" into Ukrainian legislation, as well as to activate the training of HTA experts at the hospital level
References
- The International Network of Agencies for Health Technology Assessment (INAHTA). Available at: https://www.inahta.org/
- Sampietro-Colom, L., Lach, K., Cicchetti, A., Kidholm, K., Pasternack, I., Fure, B. et al. (2015). The AdHopHTA Handbook: A Handbook of Hospital-Based Health Technology Assessment (HB-HTA). Available at: http://www.adhophta.eu/handbook
- Departament otsinky medychnykh tekhnolohii ta ratsionalnoi farmakoterapii (2019). Available at: https://www.dec.gov.ua/ua/department-omt/
- Pro zatverdzhennia Poriadku provedennia derzhavnoi otsinky medychnykh tekhnolohii (2020). Postanova KMU No. 1300. 23.12.2020. Available at: https://zakon.rada.gov.ua/laws/show/1300-2020-п#Text
- Fіlіniuk, O. M., Aleshko, D. V., Babenko, M. M., Kosiachenko, K. L., Kakhvechі, R. (2022). Decision-making regulatory framework of the introduction of health technologies at the hospitals in Ukraine. Farmatsevtychnyi Zhurnal, 1, 6–14. doi: https://doi.org/10.32352/0367-3057.1.22.01
- Filiniuk, O. M., Kosyachenko, K. L., Datsiuk, N. O., Skrylov, V. V. (2021). Health technology assessment: features of national / regional and hospital-based medical technology assessment. Social Pharmacy in Health Care, 7 (3), 21–30. doi: https://doi.org/10.24959/sphhcj.21.230
- Filiniuk, O. M., Babenko, M. M. (2023). International experience of using report forms for hospital-based health technology asssessment. Farmatsevtychnyi Zhurnal, 1, 25–32. doi: https://doi.org/10.32352/0367-3057.1.23.03
- Bodeau-Livinec, F., Simon, E., Montagnier-Petrissans, C., Joël, M.-E., Féry-Lemonnier, E. (2006). Impact of CEDIT recommendations: An example of health technology assessment in a hospital network. International Journal of Technology Assessment in Health Care, 22 (2), 161–168. doi: https://doi.org/10.1017/s0266462306050975
- Avdeyev, A., Tabarov, A., Akhetov, A., Shanazarov, N., Hailey, D., Kaptagayeva, A. et al. (2019). Hospital-based Health Technology Assessment in Kazakhstan: 3 years’ experience of one unit. International Journal of Technology Assessment in Health Care, 35 (6), 436–440. doi: https://doi.org/10.1017/s0266462318003744
- Kidholm, K., Ølholm, A. M.; Sampietro-Colom, L., Martin, J. (Eds.) (2016). Hospital-Based HTA in Denmark. Hospital-Based Health Technology Assessment. Cham: Adis. Available at: doi: https://doi.org/10.1007/978-3-319-39205-94
- Halmesmäki, E., Pasternack, I., Roine, R. (2016). Hospital-based health technology assessment (HTA) in Finland: a case study on collaboration between hospitals and the national HTA unit. Health Research Policy and Systems, 14 (1). doi: https://doi.org/10.1186/s12961-016-0095-2
- Martin, J., Polisena, J., Dendukuri, N., Rhainds, M., Sampietro-Colom, L. (2016). Local health technology assessment in Canada: current state and next steps. International Journal of Technology Assessment in Health Care, 32 (3), 175–180. doi: https://doi.org/10.1017/s0266462316000210
- Gałązka-Sobotka, M., Kowalska-Bobko, I., Lach, K., Mela, A., Furman, M., Lipska, I. (2021). Recommendations for the Implementation of Hospital Based HTA in Poland: Lessons Learned From International Experience. Frontiers in Pharmacology, 11. doi: https://doi.org/10.3389/fphar.2020.594644
- Yang, J., Joo, Y., Shin, C. (2016). Frameworks for Hospital-Based Health Technology Assessment to Resolve Financial Issues in South Korea. RRJMHS, 5 (1), 39–37. Available at: https://www.rroij.com/open-access/frameworks-for-hospitalbased-health-technology-assessment-to-resolve-financial-issues-in-south-korea-.pdf
- Tsentr proviv pershyi vebinar z hospitalnoi OMT dlia likariv (2021). Available at: https://www.dec.gov.ua/news/czentr-proviv-pershyj-vebinar-z-gospitalnoyi-omt-dlya-likariv/
- DeJonckheere, M., Vaughn, L. M. (2019). Semistructured interviewing in primary care research: a balance of relationship and rigour. Family Medicine and Community Health, 7 (2), e000057. doi: https://doi.org/10.1136/fmch-2018-000057
- On State Financial Guarantees of Medical Service to the Population (2017). Law of Ukraine No. 2168-VIII. 19.10.2017. Available at: https://zakon.rada.gov.ua/laws/show/2168-19?lang=en#Text
- On Public Procurement (2015). Law of Ukraine No. 922-VIII. 25.12.2015. Available at: https://zakon.rada.gov.ua/laws/show/922-19?lang=en#Text
- Poulin, P., Austin, L., Poulin, M., Gall, N., Seidel, J., Lafreniere, R., Scott, C. (2013). Introduction of new technologies and decision making processes: a framework to adapt a Local Health Technology Decision Support Program for other local settings. Medical Devices: Evidence and Research, 6, 185–193. doi: https://doi.org/10.2147/mder.s51384
- Martelli, N., Lelong, A.-S., Prognon, P., Pineau, J. (2013). Hospital-based health technology assessment for innovative medical devices in university hospitals and the role of hospital pharmacists: learning from international experience. International Journal of Technology Assessment in Health Care, 29 (2), 185–191. doi: https://doi.org/10.1017/s0266462313000019
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Olena Filiniuk, Mykhailo Babenko, Kostyantin Kosyachenko, Rabia Sucu

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






