The study of the stability of silver proteinate solutions prepared in pharmacies
DOI:
https://doi.org/10.15587/2519-4852.2023.289798Keywords:
extemporaneous dosage forms, titrimetric method of analysis, validation, silver proteinate, chemical stability, microbiological purityAbstract
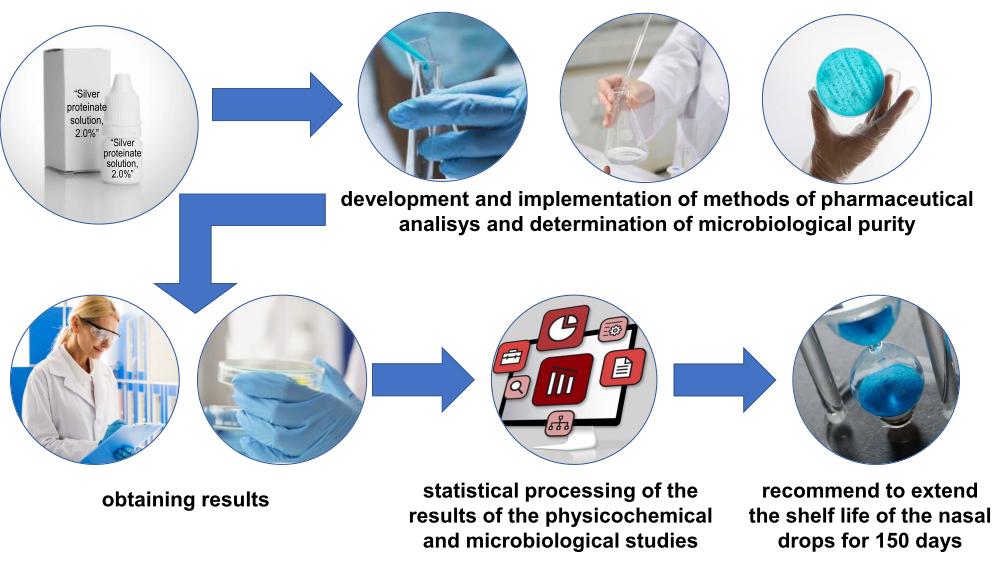
Today, medicines made in pharmacies are increasingly attracting consumers' attention and are in growing demand in Ukraine. Pharmacy production faces a number of problems, including the study of the stability of extemporaneous dosage forms and the determination of an optimal shelf life. 1 % and 2 % water silver proteinate solutions used in ophthalmology, otolaryngology and nephrology are produced in Ukrainian pharmacies both extemporaneously and as a reserve.
The aim. The aim of the work is to develop methods for quality support and study the chemical stability of 1 % and 2 % water solutions of silver proteinate, and to study the microbiological purity to extend the storage time of nasal drops.
Materials and methods. A study of the chemical stability of 1 % and 2 % water silver proteinate solutions of pharmaceutical production is carried out using chemical identification reactions (to silver and protein), quantitative determination by thiocyanatometric titrimetric method and determination of microbiological purity.
Results. The validation characteristics of the method for the quantitative thiocyanatometric determination of silver proteinate were studied (the correlation coefficient r = 0.9995 and 0.9996; the systematic error – 0.26 % and 0.03 %, the relative confidence interval – 0.88 % and 0.74 % for 1 % and 2 % solutions, respectively), as well as its suitability for this purpose was proven. “Silver proteinate solution, 1.0 %” and “Silver proteinate solution, 2.0 %” prepared in the pharmacy were studied for 150 days by the “Microbiological purity” indicator and were biologically stable.
Conclusions. The chemical identification reactions and methods for the quantitative determination of silver proteinate in an extemporaneous dosage form used to study the chemical stability of the drug have been proposed. The study results of the chemical stability and microbiological purity allow us to recommend pharmacies to extend the shelf life of nasal drops containing silver proteinate as an active pharmaceutical ingredient for 150 days
References
- Zdoryk, O. A., Georgiyants, V. A., Gryzodub, O. I., Schnatz, R. (2013). Compounding in Ukraine. International Journal of Pharmaceutical Compounding, 17 (2), 124–127.
- Savchenko, L. P., Georgiyants, V. A. (2020). Current trends in compounding of medicines and its legislative regulation in foreign countries. Farmatsevtychnyi Zhurnal, 4, 6–17. doi: https://doi.org/10.32352/0367-3057.4.20.01
- Scheepers, H. P. A., Neerup Handlos, V., Walser, S., Schutjens, M. D. B., Neef, C. (2016). Impact of the Council of Europe Resolution on quality and safety assurance requirements for medicinal products prepared in pharmacies for the special needs of patients. European Journal of Hospital Pharmacy, 24 (4), 218–223. doi: https://doi.org/10.1136/ejhpharm-2016-001017
- Scheepers, H. P., Langedijk, J., Neerup Handlos, V., Walser, S., Schutjens, M. H., Neef, C. (2016). Legislation on the preparation of medicinal products in European pharmacies and the Council of Europe Resolution. European Journal of Hospital Pharmacy, 24 (4), 224–229. doi: https://doi.org/10.1136/ejhpharm-2016-001016
- Tarnowska, M., Briançon, S., Resende de Azevedo, J., Chevalier, Y., Bolzinger, M.-A. (2020). Inorganic ions in the skin: Allies or enemies? International Journal of Pharmaceutics, 591, 119991. doi: https://doi.org/10.1016/j.ijpharm.2020.119991
- Feizi, S., Javadiyan, S., Cooksley, C. M., Shaghayegh, G., Psaltis, A. J., Wormald, P.-J., Vreugde, S. (2021). Green synthesized colloidal silver is devoid of toxic effects on primary human nasal epithelial cells in vitro. Food and Chemical Toxicology, 157, 112606. doi: https://doi.org/10.1016/j.fct.2021.112606
- Propisnova, V. V., Mіsiurova, S. V. (2020). The use of decongestants for the treatment of runny nose symptom in children: the clinical and pharmaceutical analysis of the pharmaceutical market of Ukraine. Clinical Pharmacy, 24 (3), 17–29. doi: https://doi.org/10.24959/cphj.20.1516
- Belova, V. A., Belov, O. I. (2016). Choice of silver drugs in therapy of rhinosinusitis at children age. Medical Council, 16, 60–63. doi: https://doi.org/10.21518/2079-701x-2016-16-60-63
- Lansdown, A. B. G. (2010). A Pharmacological and Toxicological Profile of Silver as an Antimicrobial Agent in Medical Devices. Advances in Pharmacological Sciences, 2010, 1–16. doi: https://doi.org/10.1155/2010/910686
- Abramson, J. J., Trimm, J. L., Weden, L., Salama, G. (1983). Heavy metals induce rapid calcium release from sarcoplasmic reticulum vesicles isolated from skeletal muscle. Proceedings of the National Academy of Sciences, 80 (6), 1526–1530. doi: https://doi.org/10.1073/pnas.80.6.1526
- Use of silver proteinate solution for prevention of severe acute respiratory syndrome, by administration to both eyes and nose (2008). Pat No. FR2853548A1. Available at: https://patents.google.com/patent/FR2853548A1/en
- Scott, J. R., Krishnan, R., Rotenberg, B. W., Sowerby, L. J. (2017). The effectiveness of topical colloidal silver in recalcitrant chronic rhinosinusitis: a randomized crossover control trial. Journal of Otolaryngology – Head & Neck Surgery, 46 (1). doi: https://doi.org/10.1186/s40463-017-0241-z
- Kanner, E. V., Usenko, D. V., Maximov, M. L., Gorelova, E. A. (2014). Modern approaches to therapy of acute rhinopharyngitis in children. RMZ,21, 1541–1543.
- Jak wycenić i wykonać krople do nosa z Protargolem (2019). Available at: https://rx.edu.pl/pytania/jak-wycenic-i-wykonac-krople-do-nosa-z-protargolem/
- Nwabudike, L. C., Tatu, A. L. (2018). Magistral Prescription With Silver Nitrate and Peru Balsam in Difficult-to-Heal Diabetic Foot Ulcers. American Journal of Therapeutics, 25 (6), e679–e680. doi: https://doi.org/10.1097/mjt.0000000000000622
- Derzhavna Farmakopeia Ukrainy. Vol. 1 (2015). Kharkiv: Derzhavne pidpryiemstvo «Ukrainskyi naukovyi farmakopeinyi tsentr yakosti likarskykh zasobiv», 1128.
- Zdoryk, A. A., Savchenko, L. P., Georgiyants, V. A., Gryzodub, A. I. (2016). The development of the monographs for compoundED preparations for the state pharmacopoeia of Ukraine. The Bulletin of the Scientific Centre for Expert Evaluation of Medicinal Products, 3, 12–15.
- European Pharmacopoeia 10th Ed. (2019). Available at: https://www.edqm.eu/en/d/85150
- GF ІX SSSR (1961). Moscow: Medgiz, 387.
- Kuleshova, M. I., Guseva, L. N., Sivitckaia, O. K. (1989). Analiz lekarstvennykh form, izgotovliaemykh v aptekakh. Moscow: Meditcina, 288.
- Japanese Pharmacopoeia 18 th Ed (2021). Available at: https://www.pmda.go.jp/english/rs-sb-std/standards-development/jp/0029.html
- Derzhavna Farmakopeia Ukrainy. Vol. 3 (2014). Kharkiv: Derzhavne pidpryiemstvo «Ukrainskyi naukovyi farmakopeinyi tsentr yakosti likarskykh zasobiv», 732.
- Yevtifieieva, O. A., Heorhiiants, V. A. (2021). Medicines manufactured in pharmacies: features of validation of analytical methods and tests (Prior to the introduction of the monograph section of the SPU). Farmatsevtychnyi Zhurnal, 6, 80–93. doi: https://doi.org/10.32352/0367-3057.6.21.08
- Derzhavna Farmakopeia Ukrainy. Vol. 3 (2014). Kharkiv: Derzhavne pidpryiemstvo «Ukrainskyi naukovyi farmakopeinyi tsentr yakosti likarskykh zasobiv», 732.
- Esamuratov, A. I., Abdalov, I. B., Abdalova, K. I. (2023). Modern options for the treatment of acute rhinosinusitis. Theory and analytical aspects of recent research, 2 (14), 20–22.
- Kolyabina, E. S., Maksimova, T. V., Pleteneva, T. V., Syroeshkin, A. V. (2023). X-ray fluorescence determination of silver in colloidal solutions. Problems of Biological, Medical And Pharmaceutical Chemistry, 26 (4), 28–31.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Valeriia Cherniakova, Nataliia Bevz, Oksana Strilets, Nataliia Harna, Olena Bevz, Olga Yevtifieieva

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






