Results of the expert survey assessing the efficiency level of the national regulatory system in the field of medicines turnover
DOI:
https://doi.org/10.15587/2519-4852.2023.290086Keywords:
national regulatory systems, national regulatory authority, drug circulation, self-diagnosisAbstract
The aim. The aim of the research is to analyze the efficiency level of the national regulatory system in the field of medicines circulation using self-diagnostic tools, which will allow identifying reserves for its optimization and further improvement.
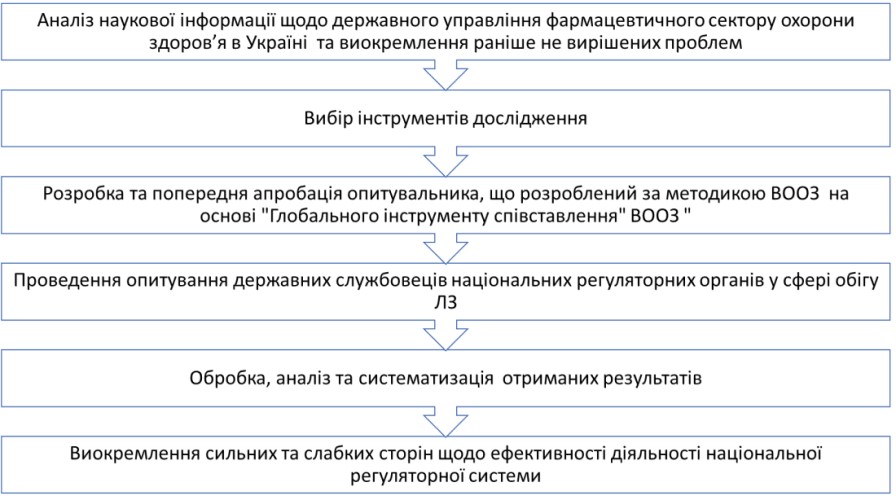
Materials and methods. The research materials consisted of the results of a survey conducted among government officials of national regulatory authorities in the field of medicines circulation. The research utilized methods of sociological survey, descriptive statistics, graphical analysis, data grouping, and generalization techniques. The research includes the development of a questionnaire based on the World Health Organization's "Global Benchmarking Tool" methodology, adapted to the national healthcare system. The questionnaire consisted of 2 questions to determine respondent characteristics and 104 statements about the functioning of the national regulatory system in the field of drug circulation.
Results. According to 75 % of the surveyed officials in Ukraine, an effective regulatory system operates in the field of drug circulation. Based on the number of affirmative answers, all statements were divided into 4 groups: high, sufficient, moderate, and low. Thus, the quality management system of national regulatory authorities was evaluated at a high level, while the funding system of national regulatory authorities received the lowest ratings. The grouping allowed us to identify weaknesses in the activity of the national regulatory system in the field of drug circulation, such as the lack of funds in budgets for staff training and overall insufficient funding for national regulators, including insufficient funding from international donors, lack of clarity and comprehensiveness in the regulatory framework for the pharmaceutical sector, etc.
Conclusions. In accordance with the aim of the article, we conducted research on the efficiency level of the national regulatory system in the field of drug circulation, which revealed that the most mature and effective direction of its activity today is the development and implementation of quality systems in national regulatory authorities
References
- Elbe, S., Roemer-Mahler, A., Long, C. (2015). Medical countermeasures for national security: A new government role in the pharmaceuticalization of society. Social Science & Medicine, 131, 263–271. doi: https://doi.org/10.1016/j.socscimed.2014.04.035
- Khovpun, O. S. (2020). Purpose, objectives and principles of public administration of pharmacia. Visnyk APSVT, 1-2, 53–63.
- Pro zatverdzhennia Derzhavnoi stratehii realizatsii derzhavnoi polityky zabezpechennia naselennia likarskymy zasobamy na period do 2025 r. (2018). Postanova KMU No. 1022. 05.12.2018. Available at: https://www.kmu.gov.ua/npas/pro-zatverdzhennarskimi-zasobami-na-period-do-2025-roku
- Lapuente, V., Van de Walle, S. (2020). The effects of new public management on the quality of public services. Governance, 33 (3), 461–475. doi: https://doi.org/10.1111/gove.12502
- Dynys, H. H., Karabin, T. O., Lazur, Ya. V., Mendzhul, M. V., Naturkach, R. P., Rohach, O. Ya. et al.; Savchyna, M. V. (Ed.) (2015). Uprovadzhennia detsentralizatsii publichnoi vlady v Ukraini: natsionalnyi i mizhnarodnyi aspekty. Uzhhorod: TIMPANI, 216.
- Tsili staloho rozvytku: Ukraina. Natsionalna dopovid (2017). Ministerstvo ekonomichnoho rozvytku ta torhivli Ukrainy. Available at: https://www.kmu.gov.ua/storage/app/sites/1/natsionalna-dopovid-csr-Ukrainy.pdf
- Lobbyists, Governments and Public Trust. Vol. 3: Implementing the OECD Principles for Transparency and Integrity in Lobbying (2014). Paris: OECD Publishing. doi: https://doi.org/10.1787/9789264214224-en
- Pro zatverdzhennia Nastanovy "Likarski zasoby. Nalezhna rehuliatorna praktyka" (2013). Nakaz MOZ Ukrainy No. 247. 28.03.2013. Available at: https://zakon.rada.gov.ua/rada/show/v0870282-13#Text
- Valentin, M. (2022). WHO Good Regulatory Practices. Available at: https://www.paho.org/sites/default/files/20220601-oms_1.pdf
- European regulatory system for medicines. Bringing new safe and effective medicines to patients across the European Union (2023). EMA. Available at: https://www.ema.europa.eu/en/documents/leaflet/european-regulatory-system-medicines_en.pdf
- WHO Global Benchmarking Tool (GBT) for evaluation of national regulatory systems (2023). World Health Organization. Available at: https://www.who.int/tools/global-benchmarking-tools
- Knysh, S. V., Gusarov, S. M., Shelukhin, N. L., Kharaberiush, I. F., Bila, V. R. (2019). Modernization of state administration system in the health care sphere of Ukraine. Wiadomości Lekarskie, 72 (5), 887–891. doi: https://doi.org/10.36740/wlek201905129
- Knysh, S. V., Yakymets, O. I. (2022). Legal regulation of the economic activities of pharmacies in Ukraine. Law and Safety, 87 (4), 184–194. doi: https://doi.org/10.32631/pb.2022.4.15
- Khovpun, O. S. (2019). Osoblyvosti zdiisnennia derzhavnoho upravlinnia u farmatsevtychnii sferi Ukrainy. Scientific Journal of Public and Private Law, 2 (5), 191–196. doi: https://doi.org/10.32844/2618-1258.2019.5-2.35
- Strelchenko, O. H. (2018). Tendentsii rozvytku zasobiv publichnoho administruvannia sfery obihu likarskykh zasobiv. Pivdennoukrainskyi pravnychyi chasopys, 4, 81–85.
- Strelchenko, O. H. (2019). Doctrinal characteristics of the subjects of public administration of the sphere of drug circulation. Public Administration and Customs Administration, 1, 7–13. doi: https://doi.org/10.32836/2310-9653-2019-1-7-13
- Aleksieiev, O. H. (2022). Perspective and current state of international standards influence on prevention and treatment of diseases through the prism of developing high-quality and safe medicines. Zaporozhye Medical Journal, 24 (4), 448–453. doi: https://doi.org/10.14739/2310-1210.2022.4.256025
- Pashkov, V., Kotvitska, A., Harkusha, A. (2017). Legal regulation of the production and trade of medical devices and medical equipment in the EU and US: experience for Ukraine. Wiadomosci Lekarskie, 70 (3 pt 2), 614–618.
- Karamyshev, D., Hordiienko, L., Lytvynenko, M. (2023). Management of the medical and evacuation system development of the armed forces of Ukraine according to NATO standards. Public Administration and Regional Development, 20, 445–470. doi: https://doi.org/10.34132/pard2023.20.08
- Karamyshev, D., Hordiienko, L., Hranovskyi, M. (2023). Aspects of management of medical and evacuation support for the troops in the conditions of the unified medical space of Ukraine. Archiv Euromedica, 12. doi: https://doi.org/10.35630/2022/12/sp.iss.5
- Kotvitska, A., Volkova, A., Korzh, I., Surikova, I. (2021). Comparative analysis of indicators that determine the effectiveness of the implementation of socio-economic determinants of health in Europe and Ukraine. ScienceRise: Pharmaceutical Science, 3 (31), 34–41. doi: https://doi.org/10.15587/2519-4852.2021.235787
- Lebed, S., Nemchenko, A., Nazarkina, V. (2020). Actuality of the implementation of international practice in proliferation of counterfeit medicines involving Interpol. Journal of Advanced Pharmacy Education and Research, 10 (2), 52–59.
- Nemchenko, A. S., Titko, I. A., Podgaina, M. V., Korzh, Y. V., Zaytzeva, Y. L. (2018). Legal and organizational economic aspects of the functioning of the main models of health-care systems. Asian Journal of Pharmaceutics, 12, 937–945.
- Nemchenko, A. S., Khomenko, V. M., Susharyna, I. V. (2018). Assessment of contemporary problems and directions of improving personnel policy in domestic pharmacy. Management, economy and quality assurance in pharmacy, 3 (55), 41–46. doi: https://doi.org/10.24959/uekj.18.21
- Susharyna, I. V., Nemchenko, A. S., Homenko, V. M. (2017). Identifying of the priority areas for improving of state and public regulation in pharmacy. Pharmaceutical Review, 1, 38–44. doi: https://doi.org/10.11603/2312-0967.2017.1.7529
- WHO Global Benchmarking Tool (GBT) for evaluation of national regulatory systems of medical products (2021). World Health Organization. Available at: https://www.who.int/publications/i/item/9789240020245
- Roth, L., Bempong, D., Babigumira, J. B., Banoo, S., Cooke, E., Jeffreys, D. et al. (2018). Expanding global access to essential medicines: investment priorities for sustainably strengthening medical product regulatory systems. Globalization and Health, 14 (1). doi: https://doi.org/10.1186/s12992-018-0421-2
- Kanavets, M., Lykhach, Yu., Kukulia, A., Butenko, O., Yerchenko, Yu.; Kuprii, V. (Ed.) (2019). Instrumenty zabezpechennia efektyvnosti, rezultatyvnosti ta yakosti diialnosti orhaniv derzhavnoi vlady. Kyiv: Tsentr adaptatsii derazhvnoi sluzhby do standartiv Yevropeiskoho Soiuzu, 178.
- Khadem Broojerdi, A., Alfonso, C., Ostad Ali Dehaghi, R., Refaat, M., Sillo, H. B. (2021). Worldwide Assessment of Low- and Middle-Income Countries’ Regulatory Preparedness to Approve Medical Products During Public Health Emergencies. Frontiers in Medicine, 8. doi: https://doi.org/10.3389/fmed.2021.722872
- Hrabovetskyi, B. Ye. (2010). Metody ekspertnykh otsinok: teoriia, metodolohiia, napriamky vykorystannia. Vinnytsia: VNTU, 171.
- Vasylenko, O. V. (2020). Komp’iuterne modeliuvannia. Zaporizhzhia: NU «Zaporizka politekhnika», 175.
- Kelsen de Oliveira, F., Brandão de Oliveira, M., Gomes, A. S., Queiros, L. M. (2019). Statistical Grouping Methods for Identifying User Profiles. International Journal of Technology and Human Interaction, 15 (2), 41–52. doi: https://doi.org/10.4018/ijthi.2019040104
- Freund, R. J., Wilson, W. J., Mohr, D. L. (2010). Data and Statistics. Statistical Methods. Academic Press, 1–65. doi: https://doi.org/10.1016/b978-0-12-374970-3.00001-9
- Khadem Broojerdi, A., Baran Sillo, H., Ostad Ali Dehaghi, R., Ward, M., Refaat, M., Parry, J. (2020). The World Health Organization Global Benchmarking Tool an Instrument to Strengthen Medical Products Regulation and Promote Universal Health Coverage. Frontiers in Medicine, 7. doi: https://doi.org/10.3389/fmed.2020.00457
- Dehaghi, R. O. A., Khadem Broojerdi, A., Paganini, L., Sillo, H. B. (2023). Collaborative training of regulators as an approach for strengthening regulatory systems in LMICs: experiences of the WHO and Swissmedic. Frontiers in Medicine, 10. doi: https://doi.org/10.3389/fmed.2023.1173291
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Maxim Suvorov, Alla Kotvitska

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






