Application of the direct encapsulation method in the technology of medicine with dry rauwolphia extract (Rauvolfia serpentina Benth.)
DOI:
https://doi.org/10.15587/2519-4852.2023.290104Keywords:
capsules, technology, direct encapsulation, Rauwolfia dry extract, hypertensionAbstract
The aim: development of the composition and technology of a medicinal drug in the form of capsules with a dry extract of Rauwolfia for the treatment of hypertension.
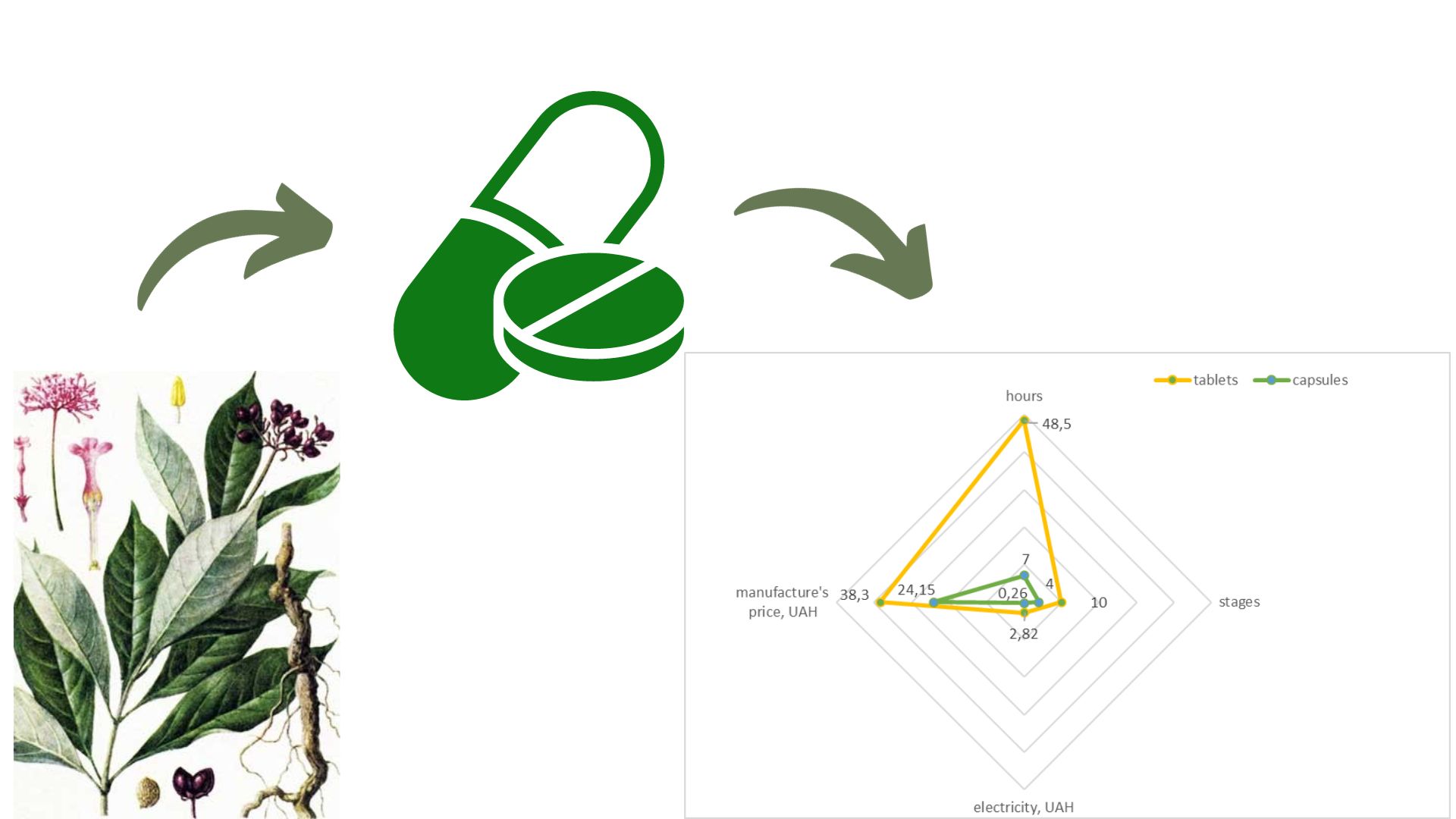
Materials and methods. Experimental samples of masses for encapsulation contained 2.0 mg of dry Rauwolfia extract and various excipients used in the technology of solid dosage forms. The study of pharmaco-technological characteristics was carried out on the devices of the company "Pharma Test" (Germany). The time of decay was determined on the device of the company "Erweka" (Germany). A comparative analysis of the cost calculation of the production of tablets and capsules with dry rauwolfia extract was carried out by the "cost plus" method.
Results. The use of PROSOLV® SMCC HD 90 improved the flowability, homogeneity and homogeneity of the mass with Rauwolfia dry extract powder, considering the low dosage of the substance of 2 mg. It has been experimentally proven that PROSOLV® SMCC HD 90 shortens the disintegration time as a disintegrant, because due to silicate moisture easily penetrates into the MCCC, hydrophilic bridges are formed, wettability increases and mass swelling occurs. The multifunctionality of PROSOLV® SMCC HD 90 three-in-one excipient, which has the properties of a filler, a disintegrant and a glidant, makes it easy to apply direct encapsulation technology, replace and reduce the number of excipients and thus increase production efficiency.
Conclusions. When developing the composition of capsules with dry rauwolfia extract, the effect of various excipients on the pharmaco-technological properties of encapsulating masses and ready-made capsules was investigated. The combined excipient, namely PROSOLV® SMCC HD 90, having the properties of a filler, a disintegrant and a lubricant, in direct encapsulation technology is more effective in influencing the fluidity of the mass and disintegration of the capsules. The introduction of the direct encapsulation method will allow to expand the range of new medicines and improve existing technologies, in particular in the form of tablets, which are widely produced in industrial production. it is possible to attach specifically to Rauwolfia
References
- Chuieshov, V. I., Hladukh, Ye. V., Saiko, I. V. et al. (2012).Tekhnolohiia likiv promyslovoho vyrobnytstva. Kharkiv: NFaU.
- Augsburger, L. (2009). Hard- and Soft-Shell Capsules. Modern Pharmaceutics: Volume 1. Basic Principles and Systems, 499–564. doi: https://doi.org/10.1201/9780824744694.ch11
- Schwedt, G. (2018). Chemieder Arzneimittel: Einfache Experimentemit Medikamentenaus der Apotheke. Mannheim: Wiley, 166.
- Qiu, Y., Chen, Y., Zhang, G. G. Z., Yu, L., Mantri, R. V. (Eds.) (2017). Developing Solid Oral Dosage Forms: Pharmaceutical Theory & Practice. San Diego.
- Augsburger, L. L., Hoag, S. W. (Eds.) (2017). Pharmaceutical Dosage Forms: Capsules. Boca Raton: CRC Press, 435. doi: https://doi.org/10.1201/9781315111896
- Fares, R., Bobrytska, L., Germanyuk, T., Kryvoviaz, O., Ivko, T., Toziuk, O. et al. (2017). Diaplant: Manufacturing technology and rationalization of costs of acute in testin al infection pharmacotherapy. International Journal of Green Pharmacy, 11 (3), 584–589. doi: https://doi.org/10.22377/ijgp.v11i03.1178
- Zlahoda, V. S., Bobrytska, L. O. (2022). Segmentation of modern dosage forms at the pharmaceutical market of Ukraine. News of Pharmacy, 104 (2), 45–48. doi: https://doi.org/10.24959/nphj.22.94
- Tverdye kapsuly: istoriia postoiannykh izmenenii i usovershenstvovanii (2013). Farmatcevticheskaia otrasl, 2 (37). Available at: http://archive.promoboz.com/n2_37/26-31.pdf
- Sovremennye dostizheniia v oblasti proizvodstva tverdykh i miagkikh kapsul. (2015). Tverdye lekarstvennye formy: kapsuly. Farmatcevticheskaia otrasl, 3 (50).
- Kinam, P. (2016). Solid dosage forms: capsule. Drug Delivery: Fundamentals and Applications. CRC Press.
- Germanyuk, T., Bobrytska, L., Ivko, T., Fares, R. (2019). Diaplant: development of technology and pharmacoeconomic evidence of therapy. Lambert Academic Publishing of International Book Market Service Ltd., 60
- Ivko, T., Aslanian, M., Bobrytska, L., Popova, N., Nazarova, O., Bereznyakova, N., Germanyuk, T. (2018). Development of the composition and manufacturing technology of the new combined drug Lavaflam. The Turkish Journal of Pharmaceutical Sciences, 15 (3), 263–270. doi: https://doi.org/10.4274/tjps.79553
- Neokardyl (Neokardil). Dovidnyk likarskykh zasobiv No. 1 v Ukraini. Available at: https://compendium.com.ua/info/173505/neokardil/
- Derzhavnyi reiestr likarskykh zasobiv Ukrainy. Available at: http://www.drlz.com.ua/
- Kovalenko, V. M. (Ed.) (2019). Dovidnyk «Kompendium 2019 – likarski preparaty». Moryon. Available at: https://compendium.com.ua/
- Derzhavna Farmakopeia Ukrainy. Vol. 1 (2015). Kharkiv: Derzhavne pidpryiemstvo «Ukrainskyi naukovyi farmatsevtychnyi tsentr yakosti likarskykh zasobiv», 1128.
- Nazarkina, V. M., Nemchenko, A. S., Kosiachenko, K. L., Babenko, M. M. (2022). Metodolohiia tsinoutvorennia na likarski zasoby v systemi okhorony zdorov’ia. Kyiv: Farmatsevt Praktyk, 288.
- Nemchenko, A. S., Nazarkina, V. M. (2015). Obgruntuvannia metodychnykh pidkhodiv do upravlinnia sobivartistiu vyrobnytstva LZ. Upravlinnia, ekonomika ta zabezpechennia yakosti v farmatsii, 3 (41), 38–43.
- Demchuk, M. B., Hroshovyi, T. A., Leskiv, O. M., Malanchuk, N. V. (2021). Comparative research of some brands of lactose as fillers for direct compression of tablets. Pharmaceutical Review, 2, 5–13. doi: https://doi.org/10.11603/2312-0967.2021.2.12056
- Ruban, O. A., Pertsev, I. M., Kutsenko, S. A., Maslii, Yu. S. (2016). Dopomizhni rechovyny u vyrobnytstvi likiv. Kharkiv: Zoloti storinky, 720.
- PROSOLV® SMCC (виробник «JRSPHARMA», USA). Available at: https://www.jrs.cn/pharma-wAssets/docs/brochures/br-prosolv-smcc-1809.pdf
- Zlahoda, V. S., Bobrytska, L. O. (2023). Bahatofunktsionalnist prosolv® smcc v tekhnolohii priamoho kapsuliuvannia. Aktualni pytannia medyko-biolohichnykh i farmatsevtychnykh nauk. Zhytomyr, 61–62.
- Rogowska, M., Iwaniak, K., Polski, A., Slawinska, K., Sobotka-Polska, K., Modrzewska, J., Poleszak, E. (2016). Influence of different excipients on the properties of hard gelatin capsules with metamizole sodium. Current Issues in Pharmacy and Medical Sciences, 29 (3), 114–117. doi: https://doi.org/10.1515/cipms-2016-0023
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Viktoriia Zlahoda, Larisa Bobrytska, Olena Nazarova, Viktoriya Tarasenko, Oleh Shpychak, Viktoriia Nazarkina, Vita Hrytsenko

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






