"Greening" of the industrial technology of enoxaparin sodium synthesis
DOI:
https://doi.org/10.15587/2519-4852.2023.290166Keywords:
enoxaparin, low molecular weight heparin, technological parameters, benzethonium salt of heparin, benzyl ester of heparin, green chemistry, e-factor, regeneration of solventsAbstract
The aim: carrying out stages of synthesis of intermediates of Enoxaparin sodium, considering the variation of synthesis parameters. Correlation analysis between the technological parameters of the synthesis and the quality of the obtained samples. Evaluation of the influence of the quality of semi-finished products on the quality of the final substance. Implementation of the principles of green chemistry in the synthesis of Enoxaparin sodium by reducing the production cycle and using the most environmentally friendly solvents and reagents.
Materials and methods: samples of intermediates of the substance Enoxaparin sodium were synthesized according to the method described in the patent, as well as with a variation of the selected critical technological parameters. The obtained samples of intermediate products were analyzed according to the internal specification. In addition, an NMR-spectroscopy analysis was carried out for detailed structural characterization of Enoxaparin sodium intermediate molecules.
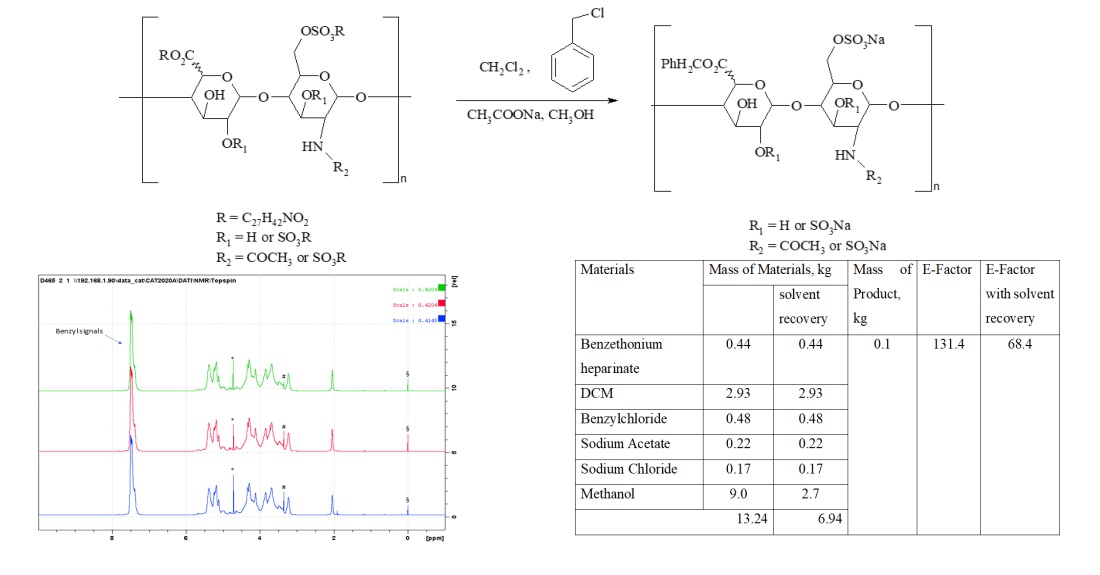
Results: the method of synthesis of intermediates of the Enoxaparin substance proposed in the patent was evaluated and the critical parameter for the formation of the final molecule was selected, namely the reaction mass holding time. The intermediate of Enoxaparin sodium - benzethonium salt of heparin and benzyl ester of heparin were developed according to the selected parameters and the analysis of the obtained samples was carried out according to the internal ND. Taking into account the principles of green chemistry, the method of synthesis of the intermediate product - benzyl ester of heparin was optimized by solvent regeneration.
Conclusions: As a result of the research, the methods of synthesis of intermediates of Enoxaparin were evaluated and the technological parameters of the synthesis of intermediates were determined, allowing to obtain a substance equivalent to the original Clexane® and Lovenox®. Evaluation of the method of synthesis of intermediate products according to the principles of green chemistry was carried out. The possibilities of greening the synthesis were analyzed. The holding time of the reaction mass of the benzethonium salt of heparin was reduced from 6 to 4 hours, and the benzyl ester of heparin from 25 to 22 hours. The E-factor indicator was reduced by regeneration of the solvent at the stage of synthesis of heparin benzyl ester
References
- Billett, H. H., Reyes-Gil, M., Szymanski, J., Ikemura, K., Stahl, L. R., Lo, Y. et al. (2020). Anticoagulation in COVID-19: Effect of Enoxaparin, Heparin, and Apixaban on Mortality. Thrombosis and Haemostasis, 120 (12), 1691–1699. doi: https://doi.org/10.1055/s-0040-1720978
- Drago, F., Gozzo, L., Li, L., Stella, A., Cosmi, B. (2020). Use of Enoxaparin to Counteract COVID-19 Infection and Reduce Thromboembolic Venous Complications: A Review of the Current Evidence. Frontiers in Pharmacology, 11. doi: https://doi.org/10.3389/fphar.2020.579886
- Iqbal, Z., Sadaf, S. (2022). Commercial Low Molecular Weight Heparins – Patent Ecosystem and Technology Paradigm for Quality Characterization. Journal of Pharmaceutical Innovation, 18 (2), 803–835. doi: https://doi.org/10.1007/s12247-022-09665-7
- Mixtures of particular LMW heparinic polysaccharides for the prophylaxis/treatment of acute thrombotic events (1995). Pat. US 5,389,618. 14.02.1995.
- Baytas, S. N., Linhardt, R. J. (2020). Advances in the preparation and synthesis of heparin and related products. Drug Discovery Today, 25 (12), 2095–2109. doi: https://doi.org/10.1016/j.drudis.2020.09.011
- Bovsunovska, Y., Rudiuk, V., Mishchenko, V., Georgiyants, V. (2023). Obtaining the substance enoxaparin sodium equivalent to the original Clexane® and Lovenox®. Selection of technological parameters of the key stage of the synthesis. ScienceRise: Pharmaceutical Science, 2 (42), 46–56. LOCKSS. doi: https://doi.org/10.15587/2519-4852.2023.277735
- de Marco, B. A., Rechelo, B. S., Tótoli, E. G., Kogawa, A. C., Salgado, H. R. N. (2019). Evolution of green chemistry and its multidimensional impacts: A review. Saudi Pharmaceutical Journal, 27 (1), 1–8. doi: https://doi.org/10.1016/j.jsps.2018.07.011
- Sheldon, R. A. (2017). Metrics of Green Chemistry and Sustainability: Past, Present, and Future. ACS Sustainable Chemistry & Engineering, 6 (1), 32–48. doi: https://doi.org/10.1021/acssuschemeng.7b03505
- Sheldon, R. A. (2017). The E factor 25 years on: the rise of green chemistry and sustainability. Green Chemistry, 19 (1), 18–43. doi: https://doi.org/10.1039/c6gc02157c
- Ivanković, A. (2017). Review of 12 Principles of Green Chemistry in Practice. International Journal of Sustainable and Green Energy, 6 (3), 39–48. doi: https://doi.org/10.11648/j.ijrse.20170603.12
- Wanisa, A. M., Qasem, A. A., Asma, O. E. (2020). Green chemistry: principles, applications, and disadvantages. Chemical Methodologies, 4, 408–423. doi: https://doi.org/10.33945/sami/chemm.2020.4.4
- Gupta, P., Mahajan, A. (2015). Green chemistry approaches as sustainable alternatives to conventional strategies in the pharmaceutical industry. RSC Advances, 5 (34), 26686–26705. doi: https://doi.org/10.1039/c5ra00358j
- Dunn, P. J. (2012). The importance of Green Chemistry in Process Research and Development. Chemical Society Reviews, 41 (4), 1452–1461. doi: https://doi.org/10.1039/c1cs15041c
- Constable, D. J. C., Jimenez-Gonzalez, C., Henderson, R. K. (2006). Perspective on Solvent Use in the Pharmaceutical Industry. Organic Process Research & Development, 11 (1), 133–137. doi: https://doi.org/10.1021/op060170h
- Prat, D., Hayler, J., Wells, A. (2014). A survey of solvent selection guides. Green Chemistry, 16 (10), 4546–4551. doi: https://doi.org/10.1039/c4gc01149j
- Alder, C. M., Hayler, J. D., Henderson, R. K., Redman, A. M., Shukla, L., Shuster, L. E., Sneddon, H. F. (2016). Updating and further expanding GSK’s solvent sustainability guide. Green Chemistry, 18 (13), 3879–3890. doi: https://doi.org/10.1039/c6gc00611f
- Prat, D., Wells, A., Hayler, J., Sneddon, H., McElroy, C. R., Abou-Shehada, S., Dunn, P. J. (2016). CHEM21 selection guide of classical- and less classical-solvents. Green Chemistry, 18 (1), 288–296. doi: https://doi.org/10.1039/c5gc01008j
- Process for the preparation of low molecular weight heparin (2018). Pat. WO2019116217A2.
- Jordan, A., Stoy, P., Sneddon, H. F. (2020). Chlorinated Solvents: Their Advantages, Disadvantages, and Alternatives in Organic and Medicinal Chemistry. Chemical Reviews, 121 (3), 1582–1622. doi: https://doi.org/10.1021/acs.chemrev.0c00709
- Byrne, F. P., Jin, S., Paggiola, G., Petchey, T. H. M., Clark, J. H., Farmer, T. J. et al. (2016). Tools and techniques for solvent selection: green solvent selection guides. Sustainable Chemical Processes, 4 (1). doi: https://doi.org/10.1186/s40508-016-0051-z
- Zhenova, A. (2020). Challenges in the development of new green solvents for polymer dissolution. Polymer International, 69 (10), 895–901. doi: https://doi.org/10.1002/pi.6072
- Rudd, T. R., Skidmore, M. A., Guimond, S. E., Cosentino, C., Torri, G., Fernig, D. G. et al. (2008). Glycosaminoglycan origin and structure revealed by multivariate analysis of NMR and CD spectra. Glycobiology, 19 (1), 52–67. doi: https://doi.org/10.1093/glycob/cwn103
- Pomin, V. H., Mulloy, B. (2021). Nuclear Magnetic Resonance Methods in Structural Characterization of Glycosaminoglycans. Glycosaminoglycans. New York, 183–207. doi: https://doi.org/10.1007/978-1-0716-1398-6_16
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Yuliia Bovsunovska, Vitalii Rudiuk, Victoriya Georgiyants

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






