Assessment of medical technologies in the formation of government programs to assist patients with rare metabolic diseases
DOI:
https://doi.org/10.15587/2519-4852.2023.290218Keywords:
health technology assessment (HTA), government programs, phenylketonuria (PKU), rare (orphan) diseases, food products for special medical purposes, special low protein foods for phenylketonuria – SLPF-PKU, medical technologies (MT), nutritional therapy (diet-for-life)Abstract
The aim: carrying out an assessment of the technologies of nutritional and pharmacological therapy of phenylketonuria (PKU) to justify a set of measures for the implementation of the government program to support patients with rare diseases (RD).
Materials and methods: scientific publications, regulatory acts, treatment protocols, statistical data, epidemiological indicators, results of patient questionnaires, marketing information, data from the electronic procurement system "ProZorro" were used in the research process. The research was conducted using the methodology of health technology assessment (HTA), methods of marketing analysis, questionnaire survey, document analysis, comparison, systematization and generalization of data.
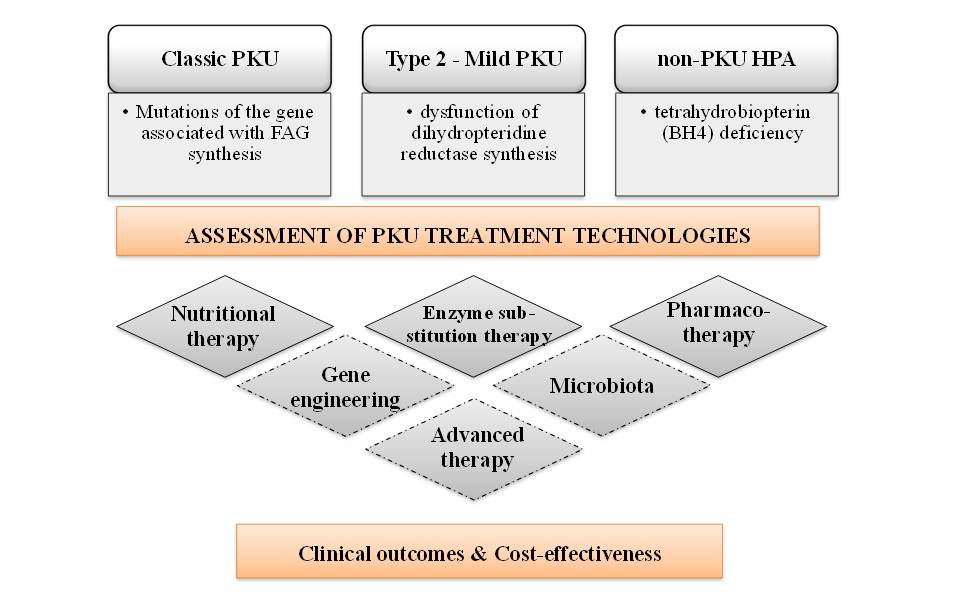
Research results. An analysis of the evaluation of modern approaches to the treatment of hereditary rare metabolic disease (PKU) was carried out. According to clinical protocols, the main technology for the treatment of PKU is nutritional therapy - a diet (diet-for-life) with restriction of the use of phenylalanine (Phe) and the use of food products for special medical purposes (Special low protein foods for phenylketonuria - SLPF-PKU). Innovative drugs "Kuvan" and "Palynziq" are recommended in the case of atypical PKU.
For RD, specific and innovative cost-effective medical technologies (MT) are usually used, which have insufficient evidence due to limited experience, low availability, and small patient populations. Centralized procurement and managed entry agreements (MEA) make it possible to expand the availability of MT to patients and obtain real data on their safety and effectiveness. Integration into the global information space, participation in international projects, joint clinical assessment (JCA) in accordance with Regulation (EU) 2021/2282 on HTA, cooperation with Orphanet, EURORDIS, other professional and patient organizations are extremely important.
The key components of HTA for RD are socio-economic and organizational and legal aspects, in particular the special status of MT, which provides certain preferences. The foreign experience of providing orphan patients (in particular, reimbursement) is summarized. The legal framework for RD is systematized.
Based on the results of the analysis of PKU prevalence indicators, modelling and budget impact calculations were carried out, considering that SLPF-PKU products are purchased from local budgets.
An analysis of prescriptions, assortment, and prices of SLPF-PKU was carried out using data from the ProZorro procurement system. A survey of 156 patients with PKU made it possible to identify unmet needs and formulate recommendations for expanding the SLPF-PKU food basket.
Conclusions: Conducting the HTA made it possible to identify key problems, as well as to justify a set of measures for the development and implementation of the government program to support patients with rare diseases, based on the obtained results
References
- Council Recommendation of 8.06.2009 on an Action in the Field of Rare Diseases (2009/C 151/02). Official Journal of the European Union. Available at: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:C:2009:151:0007:0010:EN:PDF
- Czech, M., Baran-Kooiker, A., Atikeler, K., Demirtshyan, M., Gaitova, K., Holownia-Voloskova, M. et al. (2020). A Review of Rare Disease Policies and Orphan Drug Reimbursement Systems in 12 Eurasian Countries. Frontiers in Public Health, 7. doi: https://doi.org/10.3389/fpubh.2019.00416
- Gammie, T., Lu, C. Y., Babar, Z. U.-D. (2015). Access to Orphan Drugs: A Comprehensive Review of Legislations, Regulations and Policies in 35 Countries. PLOS ONE, 10 (10), e0140002. doi: https://doi.org/10.1371/journal.pone.0140002
- Detiček, A., Locatelli, I., Kos, M. (2018). Patient Access to Medicines for Rare Diseases in European Countries. Value in Health, 21 (5), 553–560. doi: https://doi.org/10.1016/j.jval.2018.01.007
- Dharssi, S., Wong-Rieger, D., Harold, M., Terry, S. (2017). Review of 11 national policies for rare diseases in the context of key patient needs. Orphanet Journal of Rare Diseases, 12 (1). doi: https://doi.org/10.1186/s13023-017-0618-0
- Orphan Medicinal Product Designation/ Overview 2000–2022 (2023). ЕМА. Available at: https://www.ema.europa.eu/en/documents/other/orphan-medicines-figures-2000-2022_en.pdf
- Hrechanina, O. Ya., Tereshchenko, A. V., Odynetskyi, V. A., Hrechanina, Yu. B. et al. (2013). Fenilketonuriia. Klinika. Diahnostyka. Likuvannia. Kharkiv.
- Rondanelli, M., Porta, F., Gasparri, C., Barrile, G. C., Cavioni, A., Mansueto, F. et al. (2023). A food pyramid for adult patients with phenylketonuria and a systematic review on the current evidences regarding the optimal dietary treatment of adult patients with PKU. Clinical Nutrition, 42 (5), 732–763. doi: https://doi.org/10.1016/j.clnu.2023.03.007
- Couce, M. L., de Castro, M. J., de Lamas, C., Leis, R. (2019). Effects of LC-PUFA Supplementation in Patients with Phenylketonuria: A Systematic Review of Controlled Trials. Nutrients, 11 (7), 1537. doi: https://doi.org/10.3390/nu11071537
- Thomas, L., Olson, A., Romani, C. (2023). The impact of metabolic control on cognition, neurophysiology, and well-being in PKU: A systematic review and meta-analysis of the within-participant literature. Molecular Genetics and Metabolism, 138 (1), 106969. doi: https://doi.org/10.1016/j.ymgme.2022.106969
- Adaptovana klinichna nastanova, zasnovana na dokazakh. Fenilketonuriia ta inshi hiperfenilalaninemii (2015). DETs MOZ UkrainyAvailable at: https://www.dec.gov.ua/?ZG93bmxvYWQ=d3AtY29udGVudC91cGxvYWRzLzIwMTkvMTEvMjAxNV83NjBfYWtuX2Zlbmlsay5wZGY=
- Unifikovanyi klinichnyi protokol pervynnoi, vtorynnoi (spetsializovanoi) ta tretynnoi (vysokospetsializovanoi) medychnoi dopomohy. Fenilketonuriia ta inshi hiperfenilalaninemii (2015). Nakaz MOZ Ukrainy No. 760. 19.11.2015. Available at: https://cutt.ly/6UHXK5n
- Van Spronsen, F. J., van Wegberg, A. M., Ahring, K., Bélanger-Quintana, A., Blau, N., Bosch, A. M. et al. (2017). Key European guidelines for the diagnosis and management of patients with phenylketonuria. The Lancet Diabetes & Endocrinology, 5 (9), 743–756. doi: https://doi.org/10.1016/s2213-8587(16)30320-5
- Robertson, L., Adam, S., Ellerton, C., Ford, S., Hill, M., Randles, G. et al. (2022). Dietetic Management of Adults with Phenylketonuria (PKU) in the UK: A Care Consensus Document. Nutrients, 14 (3), 576. doi: https://doi.org/10.3390/nu14030576
- Pharmacy/medical policy 5.01.585 Pharmacologic Treatment of Phenylketonuria (2023). Premera НМО, АМА. Available at: https://www.premera.com/hmo/medicalpolicies/5.01.585.pdf
- Vockley, J., Andersson, H. C., Antshel, K. M., Braverman, N. E., Burton, B. K., Frazier, D. M. et al. (2014). Phenylalanine hydroxylase deficiency: diagnosis and management guideline. Genetics in Medicine, 16 (2), 188–200. doi: https://doi.org/10.1038/gim.2013.157
- The portal for rare diseases and orphan drugs. Available at: https://www.orpha.net/consor/cgi-bin/Education.php?lng=EN
- Zvit pro nadannia medyko-henetychnoi dopomohy za 2022 r. (forma No. 49). Tsentr hromadskoho zdorov’ia (2022). MOZ Ukrainy.Available at: http://medstat.gov.ua/ukr/statdanMMXIX.html
- Tutuk, V., Zagayko, A., Lytkin, D. (2020). Indicators of blood biochemical analysis in PKU patients being on amino acid mixtures substitute nutrition. Ukrainian Biopharmaceutical Journal, 3 (64), 30–37. doi: https://doi.org/10.24959/ubphj.20.278
- Nazarkina, V. M., Nemchenko, A. S., Kosiachenko, K. L., Babenko, M. M.; Nemchenko, A. S. (Ed.) (2022). Metodolohiia tsinoutvorennia na likarski zasoby v systemi okhorony zdorov’ia. Kyiv: Farmatsevt Praktyk, 288.
- Low Protein Foods available on prescription for patients with PKU (2023). NSPKU. Available at: https://nspku.org/download/low-protein-foods-available-on-prescription-for-patients-with-pku/
- Wood, G., Pinto, A., Evans, S., Daly, A., Adams, S., Costelloe, S. et al. (2021). Special Low Protein Foods Prescribed in England for PKU Patients: An Analysis of Prescribing Patterns and Cost. Nutrients, 13 (11), 3977. doi: https://doi.org/10.3390/nu13113977
- Unit Guidelines for the Prescription of Low Protein Foods (2023). NSPKU. Available at: https://nspku.org/download/unit-guidelines-for-the-prescription-of-low-protein-foods/
- Ney, D. M., Blank, R. D., Hansen, K. E. (2013). Advances in the nutritional and pharmacological management of phenylketonuria. Current Opinion in Clinical Nutrition and Metabolic Care, 17 (1), 61–68. doi: https://doi.org/10.1097/mco.0000000000000002
- Al Hafid, N., Christodoulou, J. (2015). Phenylketonuria: a review of current and future treatments. Translational Pediatrics, 4 (4), 304–317. doi: https://doi.org/10.3978/j.issn.2224-4336.2015.10.07
- Wiedemann, A., Oussalah, A., Jeannesson, É., Guéant, J.-L., François, F. (2020). Phenylketonuria, from diet to gene therapy. Medical Sciences, 36 (8-9), 725–734. doi: https://doi.org/10.1051/medsci/2020127
- PKU Golike: the evolution in PKU nutritional management. Available at: https://metahealthcare.co.uk/product-page/
- Van Wegberg, A. M. J., MacDonald, A., Ahring, K., Bélanger-Quintana, A., Blau, N., Bosch, A. M. et al. (2017). The complete European guidelines on phenylketonuria: diagnosis and treatment. Orphanet Journal of Rare Diseases, 12 (1). doi: https://doi.org/10.1186/s13023-017-0685-2
- Pena, M. J., Almeida, M. F., van Dam, E., Ahring, K., Bélanger-Quintana, A., Dokoupil, K. et al. (2015). Special low protein foods for phenylketonuria: availability in Europe and an examination of their nutritional profile. Orphanet Journal of Rare Diseases, 10 (1). doi: https://doi.org/10.1186/s13023-015-0378-7
- MacDonald, A., van Wegberg, A. M. J., Ahring, K., Beblo, S., Bélanger-Quintana, A., Burlina, A. et al. (2020). PKU dietary handbook to accompany PKU guidelines. Orphanet Journal of Rare Diseases, 15 (1). doi: https://doi.org/10.1186/s13023-020-01391-y
- Nazarkina, V., Tutuk, V. (2023). Analysis of the supply of products for special medical purposes to children with phenylketonuria. Health & Education, 1, 50–55. doi: https://doi.org/10.32782/health-2023.1.10
- Tutuk, V. V., Nazarkina, V. M. (2023). Analysis of the availability of health technologies for the treatment of phenylketonuria in Ukraine and the world. Social Pharmacy in Health Care, 9 (1), 30–38. doi: https://doi.org/10.24959/sphhcj.23.278
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Volodimir Tutuk, Viktoriia Nazarkina, M Babenko, Alla Nemchenko, Kairat Zhakipbekov

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






