Development and validation of a method for simultaneous quantitative determination of albendazole and praziquantel in coated tablets “AP-helmin”
DOI:
https://doi.org/10.15587/2519-4852.2024.290350Keywords:
validation, liquid chromatography, quantitative analysis, albendazole, praziquantel, coated tabletsAbstract
Adapting modern methods of quantitative analysis of active substances in their joint content in the dosage form and validating them is an integral process of pharmaceutical development. We have developed a drug in the form of coated tablets for the treatment of helminthiases of the digestive system in adults. A feature of this drug is the composition of the API of albendazole and praziquantel in a ratio of 1:4.
The aim of this research is to develop methodology for quantitative analysis of both substances by the method of liquid chromatography, determination of their possible mutual influence on the process, as well as validation of the proposed methods.
Materials and methods. To meet the research's set purpose, the following tasks were identified: choosing the most rational method for the quantitative determination of albendazole and praziquantel; confirming the absence of the mutual influence of APIs on the results obtained; and validating the selected methods of albendazole and praziquantel analysis.
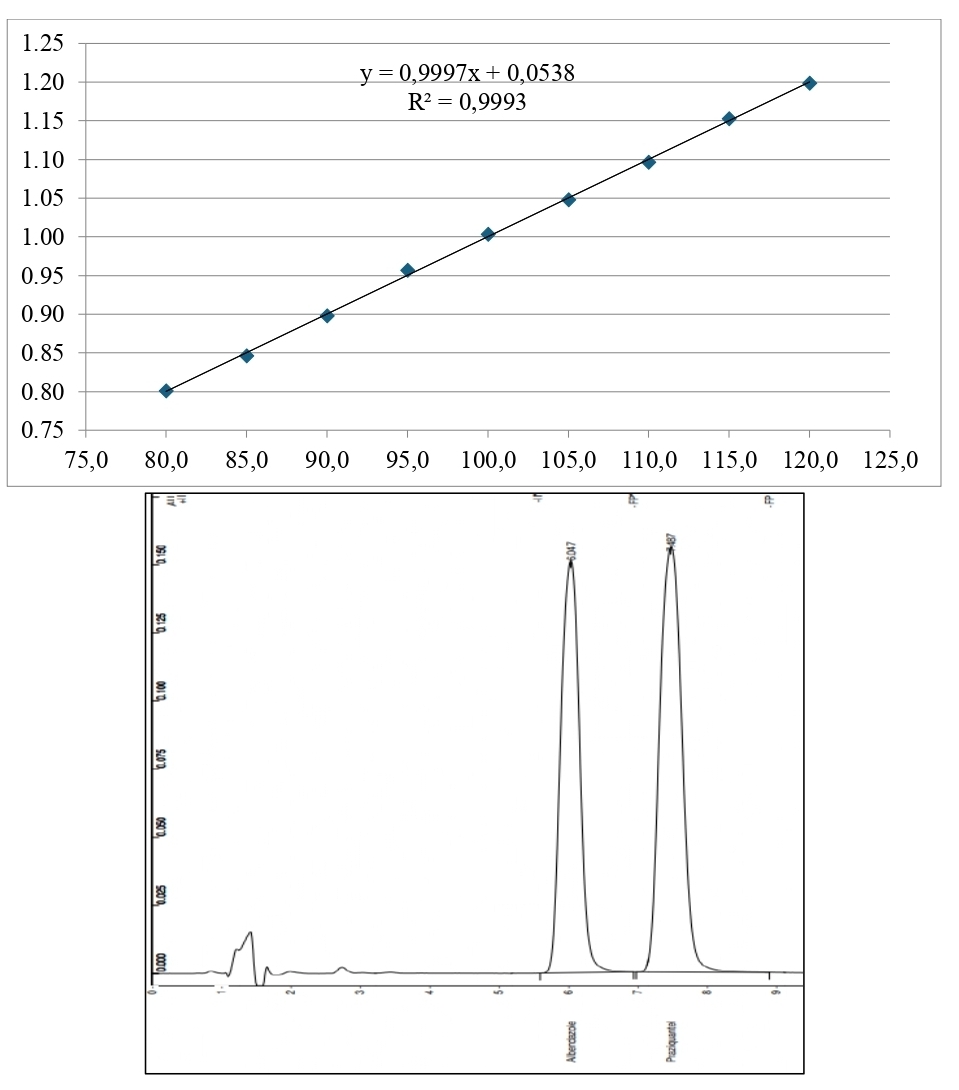
Object of the research conducted included coated tablets “AP-helmin”, series 1-5.2021; pharmacopoeial standard sample (PSS) of albendazole, and PSS praziquantel. Quantitative determination of albendazole and praziquantel was conducted according to SPU, method 2.2.29.
Results. The article describes the conditions and stages of the quantitative determination of albendazole and praziquantel and the main indicators of method validation.
Conclusions. It was proven that quantification with the liquid chromatography method of both substances is validated, and the substances do not affect each other's analysis in the coated tablets "AP-helmin" following the project of QCM for this drug. All calculated parameters meet the required validation criteria
References
- Semchenko, K., Vyshnevska, L., Polovko, N. (2018). Study of anthelmintic activity and acute toxicity of medicine of combined composition. ScienceRise: Pharmaceutical Science, 6 (16), 8–12. https://doi.org/10.15587/2519-4852.2018.151428
- Semchenko, K., Vyshnevska, L. (2020). Study of the specific activity of anthelmintic drug “Ap-helmin.” Ukrainian Biopharmaceutical Journal, 2 (63), 54–57. https://doi.org/10.24959/ubphj.20.271
- Semchenko, K. V., Vyshnevska, L. I. (2019). Pat. No. 124898 UA. Antyhelmintnyi zasib na osnovi albendazolu ta prazykvantelu. MPK: A61K 31/4184, A61K 31/47, A61P 33/00. No. a201910236; declareted: 07.10.2019, published: 08.12.2021, Bul. No. 49.
- European Pharmacopoeia (2021). Strasbourg: European Directorate for the Quality of Medicines & Health Care. Available at: https://pheur.edqm.eu/subhome/10-8
- State Pharmacopoeia of Ukraine. Vol. 1 (2015). Kharkiv: State Enterprise Ukrainian scientific pharmacopoeial centre of quality of drugs, 1128.
- Anil, W., Subhash, G., Roshan, I., Badri, P. N. (2008). Validated liquid chromatographic method for simultaneous estimation of albendazole and ivermectin in tablet dosage form. Indian Journal of Chemical Technology, 15, 617–620.
- Khanji, M., Kawas, G., Haroun, M., Rasheed, M. A., Sakur, A. A. (2020). Quantitative determination of albendazole forced degradation percentages by densitometric thin layer chromatographic method. Research Journal of Pharmacy and Technology, 13 (5), 2207–2213. https://doi.org/10.5958/0974-360x.2020.00396.0
- Kumari, N., Singh, B. (2019). Quality By Design: A systematic approach for the development of analytical method validation. Journal of Drug Delivery and Therapeutics, 9, 1006–1012.
- Fregonezi-Nery, M. M., Baracat, M. M., Kedor-Hackmann, É. R. M., Pinheiro, R. M. (2001). Determination of albendazole in oral suspension. Analytical Letters, 34 (8), 1255–1263. https://doi.org/10.1081/al-100104151
- Swamy, N., Basavaiah, K. (2014). Simple and rapid spectrophotometric assay of albendazole in pharmaceuticals using iodine and picric acid as CT complexing agents. Brazilian Journal of Pharmaceutical Sciences, 50 (4), 839–850. https://doi.org/10.1590/s1984-82502014000400019
- Refat, M. S., Mohamed, G. G., Fathi, A. (2011). Spectrophotometric Determination of Albendazole Drug in Tablets: Spectroscopic Characterization of the Charge‐transfer Solid Complexes. Chinese Journal of Chemistry, 29 (2), 324–332. https://doi.org/10.1002/cjoc.201190086
- Tella, A. C., Olabemiwo, O. M., Malwi, M. O., Obiyenwal, G. K. (2010). Developing a spectrophotometric method for the estimation of albendazole in solid and suspension forms. International Journal of Physical Sciences, 5, 379–382.
- Ahmed, D. A., Abdel-Aziz, O., Abdel-Ghany, M., Weshahy, S. A. (2018). Stability indicating determination of Albendazole in bulk drug and pharmaceutical dosage form by chromatographic and spectrophotometric methods. Future Journal of Pharmaceutical Sciences, 4 (2), 161–165. https://doi.org/10.1016/j.fjps.2018.02.001
- Ferencz, E., Kelemen, É.-K., Obreja, M., Sipos, E., Vida, S., Urkon, M., Szabó, Z.-I. (2021). Computer-assisted UHPLC method development and optimization for the determination of albendazole and its related substances. Journal of Pharmaceutical and Biomedical Analysis, 203, 114203. https://doi.org/10.1016/j.jpba.2021.114203
- Saini, G., Singh, B., Vyas, M., Durgapal, S., Rangra, N., Suttee, A. (2024). RP-HPLC method development and validation of Albendazole and its impurity. BIO Web of Conferences, 86, 01046. https://doi.org/10.1051/bioconf/20248601046
- Rashed, N. S., Zayed, S., Abdelazeem, A., Fouad, F. (2020). Development and validation of a green HPLC method for the analysis of clorsulon, albendazole, triclabendazole and ivermectin using monolithic column: Assessment of the greenness of the proposed method. Microchemical Journal, 157, 105069. https://doi.org/10.1016/j.microc.2020.105069
- Varghese, S., Vasanthi, P., Ravi, T. (2011). Simultaneous densitometric determination of ivermectin and albendazole by high-performance thin-layer chromatography. Journal of Planar Chromatography – Modern TLC, 24 (4), 344–347. https://doi.org/10.1556/jpc.24.2011.4.13
- Saleh, H., Schnekenburger, J. (1992). Determination of praziquantel and of praziquantel in tablets by gas–liquid chromatography. The Analyst, 117 (9), 1457–1460. https://doi.org/10.1039/an9921701457
- Mainardes, R. M., Cinto, P. O., Gremião, M. P. D. (2006). High-performance Liquid Chromatography Determination of Praziquantel in Tablets and Raw Materials. Acta Farmacutica Bonaerense, 25 (4), 567–570.
- Soto, C., Contreras, D., Orellana, S., Yañez, J., Toral, M. I. (2010). Simultaneous Determination of Albendazole and Praziquantel by Second Derivative Spectrophotometry and Multivariated Calibration Methods in Veterinary Pharmaceutical Formulation. Analytical Sciences, 26 (8), 891–896. https://doi.org/10.2116/analsci.26.891
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Kateryna Semchenko, Liliia Vyshnevska, Volodymyr Iakovenko, Tetyana Kovalova, Mykhailo Marchenko, Yelizaveta Zuikina, Yana Marchenko

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






