Greening of the method for simultaneous determining the enisamium iodide and tilorone dihydrochloride using GC-FID assay
DOI:
https://doi.org/10.15587/2519-4852.2023.295120Keywords:
Enisamium iodide, Tilorone dihydrochloride, GC-FID, method development, validation, “green” analytical analysis, pharmaceutical wastesAbstract
Pharmaceutical companies in Ukraine aspire to develop their innovative medicinal products and successfully introduce them to the global market. However, along with the prospects of increased usage of these pharmaceuticals, there arises a challenge of heightened waste production, making them a part of the over twenty million tons of PPCPs produced annually. Consequently, one of the tasks in producing new pharmaceuticals is the development of methodologies and approaches not only for quality control but also for their determination in the environment matrices.
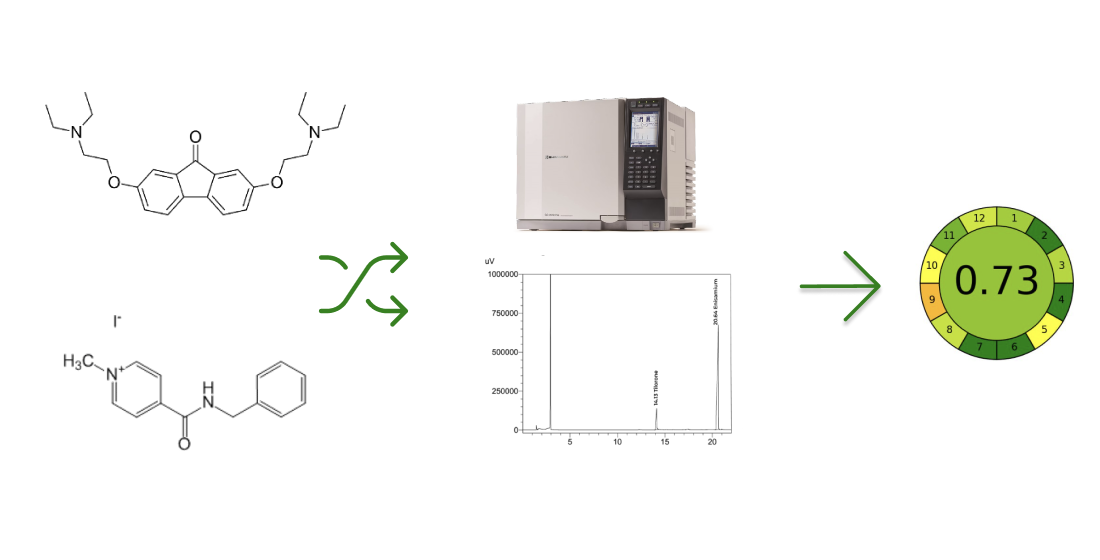
The aim. Develop and validate GC-FID chromatographic method for the simultaneous determination of Enisamium iodide and Tilorone dihydrochloride, evaluate their applicability, and compare their "greenness" with the previously developed HPLC method.
Materials and methods. The determination of the Tilorone dihydrochloride and Enisamium iodide was carried out by gas chromatography with a flame ionization detector using the Rxi-5 ms (30 m long, 0.25 mm outer diameter and 0.25 μm liquid stationary phase thickness)
Results. Chromatographic GC-FID methods have been developed for the simultaneous determination of Enisamium iodide and Tilorone dihydrochloride. Optimal sample preparation conditions were established, and a validation process was conducted. A comparison with the previously developed HPLC method was made regarding "greenness."
Conclusions. The developed GC-FID methodology is accurate and more environmentally friendly compared to the previously established methods. It can be recommended to determine Enisamium iodide and Tilorone dihydrochloride in the environmental matrices. It is considered environmentally friendly based on the overall GREENness (AGREE) scale, scoring 0.73 (>0.70), which demonstrates the environmentally favourable nature of the proposed analytical approach
References
- Cocking, D., Cinatl, J., Boltz, D. A., Peng, X., Johnson, W., Muzzio, M. et al. (2018). Antiviral effect of a derivative of isonicotinic acid enisamium iodide (FAV00A) against influenza virus. Acta Virologica, 62 (2), 191–195. doi: https://doi.org/10.4149/av_2018_211
- Haltner-Ukomadu, E., Gureyeva, S., Burmaka, O., Goy, A., Mueller, L., Kostyuk, G., & Margitich, V. (2018). In Vitro Bioavailability Study of an Antiviral Compound Enisamium Iodide. Scientia Pharmaceutica, 86 (1), 3. doi: https://doi.org/10.3390/scipharm86010003
- te Velthuis, A. J. W., Zubkova, T. G., Shaw, M., Mehle, A., Boltz, D., Gmeinwieser, N. et al. (2021). Enisamium Reduces Influenza Virus Shedding and Improves Patient Recovery by Inhibiting Viral RNA Polymerase Activity. Antimicrobial Agents and Chemotherapy, 65 (4). doi: https://doi.org/10.1128/aac.02605-20
- Ratan, R. R., Siddiq, A., Aminova, L., Langley, B., McConoughey, S., Karpisheva, K. et al. (2008). Small Molecule Activation of Adaptive Gene Expression: Tilorone or Its Analogs Are Novel Potent Activators of Hypoxia Inducible Factor-1 That Provide Prophylaxis against Stroke and Spinal Cord Injury. Annals of the New York Academy of Sciences, 1147 (1), 383–394. Portico. doi: https://doi.org/10.1196/annals.1427.033
- Tramice, A., Arena, A., De Gregorio, A., Ottanà, R., Maccari, R., Pavone, B., Arena, N., Iannello, D., Vigorita, M. G., Trincone, A. (2008). Facile Biocatalytic Access to 9‐Fluorenylmethyl Polyglycosides: Evaluation of Antiviral Activity on Immunocompetent Cells. ChemMedChem, 3 (9), 1419–1426. doi: https://doi.org/10.1002/cmdc.200800086
- Ekins, S., Lane, T. R., Madrid, P. B. (2020). Tilorone: a Broad-Spectrum Antiviral Invented in the USA and Commercialized in Russia and beyond. Pharmaceutical Research, 37 (4). doi: https://doi.org/10.1007/s11095-020-02799-8
- Gupta, D. K., Gieselmann, V., Hasilik, A., Figura, K. (1984). Isolation of the lysosomal cysteine protease cathepsin L from bovine spleen and preparation of its derivatives. Physiological chemistry, 365 (8), 859–866.
- Ekins, S., Lingerfelt, M. A., Comer, J. E., Freiberg, A. N., Mirsalis, J. C., O’Loughlin, K. et al. (2018). Efficacy of Tilorone Dihydrochloride against Ebola Virus Infection. Antimicrobial Agents and Chemotherapy, 62 (2). doi: https://doi.org/10.1128/aac.01711-17
- Chilamakuri, R., Agarwal, S. (2021). COVID-19: Characteristics and Therapeutics. Cells, 10 (2), 206. doi: https://doi.org/10.3390/cells10020206
- Kalyuzhin, O. V., Isaeva, E. I., Vetrova, E. N., Chernysheva, A. I., Ponezheva, L. O., Karaulov, A. V. (2021). Effect of Tilorone on the Dynamics of Viral Load and the Levels of Interferons and Interleukin-1β in the Lung Tissue and Blood Serum of Mice with Experimental Influenza. Bulletin of Experimental Biology and Medicine, 171 (6), 736–740. doi: https://doi.org/10.1007/s10517-021-05306-0
- Geisler, B. P., Zahabi, L., Lang, A. E., Eastwood, N., Tennant, E., Lukic, L. et al. (2021). Repurposing existing medications for coronavirus disease 2019: protocol for a rapid and living systematic review. Systematic Reviews, 10 (1). doi: https://doi.org/10.1186/s13643-021-01693-7
- Ebele, A. J., Abou-Elwafa Abdallah, M., Harrad, S. (2017). Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerging Contaminants, 3 (1), 1–16. doi: https://doi.org/10.1016/j.emcon.2016.12.004
- Chaturvedi, P., Shukla, P., Giri, B. S., Chowdhary, P., Chandra, R., Gupta, P., Pandey, A. (2021). Prevalence and hazardous impact of pharmaceutical and personal care products and antibiotics in environment: A review on emerging contaminants. Environmental Research, 194, 110664. doi: https://doi.org/10.1016/j.envres.2020.110664
- Hao, C., Lissemore, L., Nguyen, B., Kleywegt, S., Yang, P., Solomon, K. (2005). Determination of pharmaceuticals in environmental waters by liquid chromatography/electrospray ionization/tandem mass spectrometry. Analytical and Bioanalytical Chemistry, 384 (2), 505–513. doi: https://doi.org/10.1007/s00216-005-0199-y
- Water. European Commission. Available at: https://ec.europa.eu/environment/water/water-use/pharmaceuticals_en.htm
- Burmaka, O. V., Hureyeva, S. N., Marhitich, V. M. (2017). Development of HPLC method for the determination of related substances in API enisamium iodide. Farmakom, 3, 17–25.
- Burmaka, O. V., Hureieva, S. M., Marhitych, V. M. (2018). Validation of the method for determination of related impurities in the active antiviral ingredient of enisamium iodide. Zaporozhye Medical Journal, 5. doi: https://doi.org/10.14739/2310-1210.2018.5.141718
- Baktiyar, M. Z., Ishaq, B. M., Reddy L, S. S., Sreenivasulu, M. (2021). Method Development and Validation for Estimation of related Substances in Tilorone Dihydrochloride using RP¬-HPLC. Research Journal of Pharmacy and Technology, 14 (6), 3319–3324. doi: https://doi.org/10.52711/0974-360x.2021.00577
- Krasnykh, L. M., Savchenko, A. Iu., Ramenskaia, G. V. (2020). Sravnitelnoe farmakokineticheskoe izuchenie preparatov tilorona s pomoshchiu razrabotannoi metodiki VEZhKh IKF NTc ESMP, MMA im. Sechenova. Moscow.
- Belikova, A., Materienko, A., Sidorenko, L., Chorna, O., Burdulis, D., Georgiyants, V. (2022). Development of a method for the detection of amixin and amizon by HPLC on SunFire C18 column. Chemija, 33 (3), 79–86. doi: https://doi.org/10.6001/chemija.v33i3.4750
- Daughton, C. G. (2004). Pharmaceuticals and personal care products (PPCPs) as environmental pollutants: Pollution from personal actions. California Bay-Delta Authority Contaminant Stressor Workshop. Sacramento.
- International Conference on Harmonization (ICH) of Technical Requirements for the Registration of Pharmaceuticals for Human Use, Q3C (R5) (2011). Impurities: Guideline for Residual Solvents. Step 4.
- Raynie, D., Driver, J. L. (2009). Green assessment of chemical methods. Proceedings of the 13th Green Chemistry & Engineering Conference. Washigton.
- Pena-Pereira, F., Wojnowski, W., Tobiszewski, M. (2020). AGREE –Analytical GREEnness Metric Approach and Software. Analytical Chemistry, 92 (14), 10076–10082. doi: https://doi.org/10.1021/acs.analchem.0c01887
- Belikova, A., Materienko, A., Sidorenko, L., Chornyi, V., Korzh, I., Kucherenko, L. et al. (2022). Development of a method for determining the morpholinium thiazotate using more economic and green GC/MS assay with an fid detector. ScienceRise: Pharmaceutical Science, 3 (37), 4–11. doi: https://doi.org/10.15587/2519-4852.2022.259879
- European Chemicals Agency. Available at: https://echa.europa.eu/
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Anastasiia Belikova, Liudas Ivanauskas, Lyudmila Sidorenko, Vasyl Chorny, Anna Kononenko, Alla Koval, Victoriya Georgiyants

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






