Conducting biological tests in the development of self-emulsifying drug delivery systems with simvastatin
DOI:
https://doi.org/10.15587/2519-4852.2023.295450Keywords:
self-emulsifying drug delivery systems, increased activity, improved solubility, simvastatin, biological testsAbstract
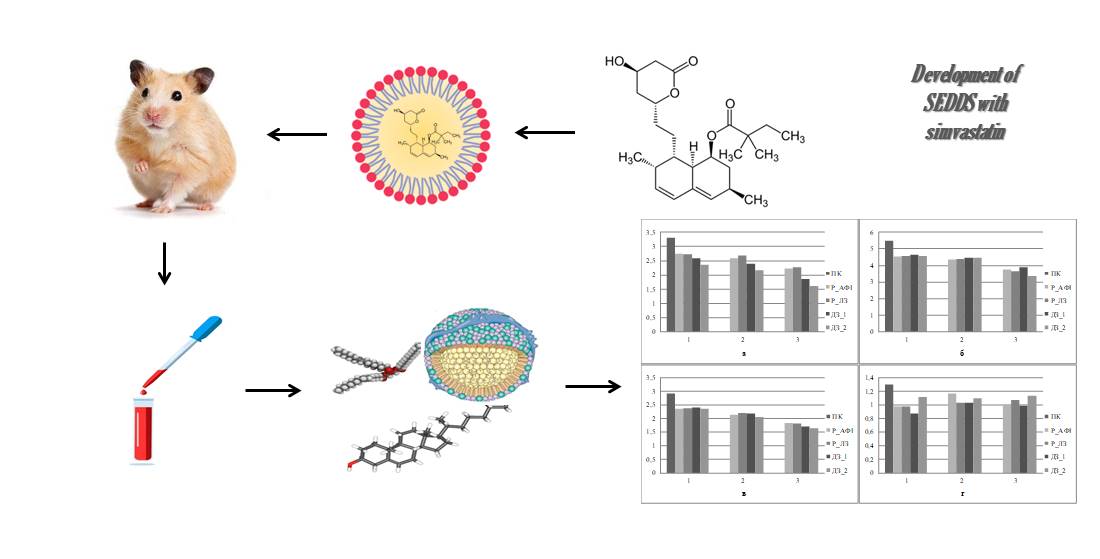
The aim of the study was to compare the hypolipidemic activity of the developed self-emulsifying drug delivery systems with simvastatin with reference samples of the substance and the finished drug product of industrial production.
Materials and methods. The substance of simvastatin (India, s. DK40-2005021, 99.09 %, introduced into the composition of self-emulsifying compositions based on castor oil (Ukraine), polyethylene glycol 40 hydrogenated castor oil (India), Tween 80 (Ukraine), glycerol monostearate (Gustav Heess GmbH, Germany) or polyethylene glycol 100 stearate (ERCA, Italy). Reference samples were Simvastatin-Sandoz (Salutas Pharma, Germany, series LX5161) and simvastatin in pure form.
The experimental animals were Syrian hamsters aged 2 months. Hyperlipidemia was modeled by means of an alimentary load. To assess the state of lipid metabolism in animals, the content of triacylglycerols, total cholesterol, low-density lipoprotein and high-density lipoprotein in the blood serum was determined by colorimetric enzymatic methods using the appropriate standard reagent kits "Triacylglycerols F" HP022.02, "Cholesterol F" HP026.02, "Cholesterol-LDL F" HP026.05 and "Cholesterol-HDL F" HP026.04 (LLC SPE "Filicit-Diagnostics", Ukraine) on a semi-automatic biochemical analyzer MapLab Plus (BSI, Italy).
Results. The reference samples had similar dose-dependent efficacy parameters. At the same time, the test samples, also having similar dose-dependent effects, in absolute terms at the maximum concentration reduced the amount of low-density lipoprotein and total cholesterol more effectively than the reference samples. When using the test samples in their average concentration, the level of triglycerols was significantly reduced, which is rather a concomitant effect of simvastatin.
Conclusions. The improvement of the overall efficacy of simvastatin when it is introduced into self-emulsifying drug delivery systems has been proved, which is associated with the modification of pharmacokinetic parameters by improving the solubility of the substance in the aqueous environment of the gastrointestinal tract
References
- Bist, V. L., Faruk, A. (2023). Recent Advancement in Self Emulsifing Drug Delivery System. Journal for Research in Applied Sciences and Biotechnology, 2 (2), 89–101. doi: https://doi.org/10.55544/jrasb.2.2.14
- Menzel, C., Holzeisen, T., Laffleur, F., Zaichik, S., Abdulkarim, M., Gumbleton, M., Bernkop-Schnürch, A. (2018). In vivo evaluation of an oral self-emulsifying drug delivery system (SEDDS) for exenatide. Journal of Controlled Release, 277, 165–172. doi: https://doi.org/10.1016/j.jconrel.2018.03.018
- Umeyor, C., Abonyi, J. I., Uronnachi, E., Salome Amarachi, C., Kenechukwu, F., Attama, A., Ibezim, E. (2020). Pharmacokinetics and Bio-Distribution Properties of a Self-Emulsifying Drug Delivery System Containing Nevirapine. Journal of Drug Discovery, Development and Delivery, 6 (1), 1035.
- Ugwu, C. E., Obitte, N. C., Onyishi, V. I., Kalombo, M. L., Onunkwo, G.C. (2016). Self- microemulsifying drug delivery system as a promising approach to improve the poorsolubility of artemether. Transylvania Review, 24 (9).
- Chen, L., Lin, X., Fan, X., Lv, Q., Fang, H., Chenchen, Y., Teng, H. (2020). A self-emulsifying formulation of Sonchus oleraceus Linn for an improved anti-diabetic effect in vivo. Food & Function, 11 (2), 1225–1229. doi: https://doi.org/10.1039/c9fo00772e
- Bodnar, L. A., Polovko, N. P. (2023). The study on the development of self-emulsifying compositions with simvastatin. News of Pharmacy, 105 (1), 32–37. doi: https://doi.org/10.24959/nphj.23.104
- Bodnar, L., Polovko, N., Bevz, N., Hrudko, V., Perepelytsia, O. (2023). Biopharmaceutical justification of the creation of self-emulsifying drug delivery systems with simvastatin. ScienceRise: Pharmaceutical Science, 2 (42), 4–10. doi: https://doi.org/10.15587/2519-4852.2023.277351
- Dalbøge, L. S., Pedersen, P. J., Hansen, G., Fabricius, K., Hansen, H. B., Jelsing, J., Vrang, N. (2015). A Hamster Model of Diet-Induced Obesity for Preclinical Evaluation of Anti-Obesity, Anti-Diabetic and Lipid Modulating Agents. PLOS ONE, 10 (8), e0135634. doi: https://doi.org/10.1371/journal.pone.0135634
- Yin, W., Carballo-Jane, E., McLaren, D. G., Mendoza, V. H., Gagen, K., Geoghagen, N. S. et al. (2012). Plasma lipid profiling across species for the identification of optimal animal models of human dyslipidemia. Journal of Lipid Research, 53 (1), 51–65. doi: https://doi.org/10.1194/jlr.m019927
- Kozhemiakin, Yu. M., Khromov, O. S., Filonenko, M. A., Saifetdinova, H. A. (2002). Naukovo-praktychni rekomendatsii z utrymannia laboratornykh tvaryn ta roboty z nymy. Kyiv: Derzhavnyi farmakolohichnyi tsentr MOZ Ukrainy, 155.
- Directive (EU) 2010/63/EU of the European Parliament and of the Council of 22 September 2010 On the Protection of Animals Used for Scientific Purposes (2010). Official Journal of the European Union, 276, 33–79.
- Lee, L.-C., Wei, L., Huang, W.-C., Hsu, Y.-J., Chen, Y.-M., Huang, C.-C. (2015). Hypolipidemic Effect of Tomato Juice in Hamsters in High Cholesterol Diet-Induced Hyperlipidemia. Nutrients, 7 (12), 10525–10537. doi: https://doi.org/10.3390/nu7125552
- Nair, A., Jacob, S. (2016). A simple practice guide for dose conversion between animals and human. Journal of Basic and Clinical Pharmacy, 7 (2), 27–31. doi: https://doi.org/10.4103/0976-0105.177703
- Symvastatyn Sandoz®. Normatyvno-derektyvni dokumenty MOZ Ukrainy. Available at: https://mozdocs.kiev.ua/likiview.php?id=47332 Last accessed: 08.06.2023
- Derzhavna farmakopeia Ukrainy. Vol. 2 (2014). Kharkiv: DP «Ukrainskyi naukovyi tsentr yakosti likarskykh zasobiv», 724.
- European Department for the Quality of Medicines. (2013). European Pharmacopoeia. Strasbourg, 3655.
- The MHLW Ministerial Notification No. 220 (2021). The Japanese Pharmacopoeia. Tokyo, 2587.
- Indrayan, A., Malhotra, K. R. (2018). Medical biostatistics. Boca Raton: CRC Press, 685.
- Schachter, M. (2005). Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundamental & Clinical Pharmacology, 19 (1), 117–125. doi: https://doi.org/10.1111/j.1472-8206.2004.00299.x
- Li, Z., Zhang, J., Zhang, Y., Zhou, L., Zhao, J., Lyu, Y. et al. (2020). Intestinal absorption and hepatic elimination of drugs in high‐fat high‐cholesterol diet‐induced non‐alcoholic steatohepatitis rats: exemplified by simvastatin. British Journal of Pharmacology, 178 (3), 582–599. doi: https://doi.org/10.1111/bph.15298
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Liubov Bodnar, Dmytro Lytkin, Nataliia Polovko

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






