Study on content of flavonoids and antioxidant activity of the raw materials of Parthenocissus quinquefolia (L.) Planch.
DOI:
https://doi.org/10.15587/2519-4852.2023.295506Keywords:
Parthenocissus quinquefolia, leaves, shoots, fruits, polyphenol compounds, flavonoids, catechins, rutin, HPLС, antioxidant activityAbstract
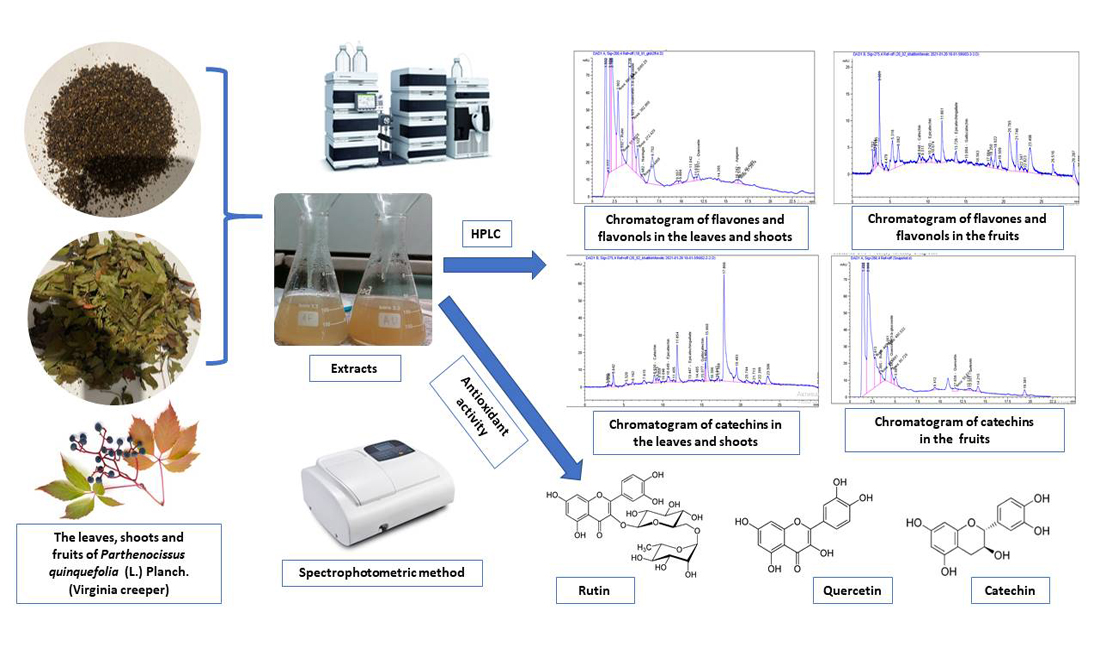
The aim of this work was to determine the component composition and quantitative content of flavonoids, including catechins, in the leaves, shoots and fruits of Virginia creeper (Parthenocissus quinquefolia (L.) Planch.) for further standardization of raw materials and establishing antioxidant activity of their water-ethanol extracts.
Materials and methods. The leaves, shoots of Parthenocissus quinquefolia were collected during the phase of mass flowering in July 2020 and fruits were collected during full ripeness (September – October 2020) in Lisnyky village of Obukhiv district of Kyiv Oblast (Ukraine). Determination of the component composition and quantitative content of flavonoids, including catechins, was carried out by the method of high-performance liquid chromatography (HPLC) on an Agilent Technologies 1200 liquid chromatograph. Identification and quantitative analysis were carried out using standard solutions of flavonoids (rutin, quercetin-3-b-glycoside, naringin, neohesperidin, quercetin, naringenin, kaempferol, luteolin, apigenin) and catechins (pyrocatechin, catechin, epicatechin, epicatechin gallate and halocatechin). Antioxidant activity was determined by the spectrophotometric method at 347 nm by the ability to inhibit the autooxidation of adrenaline in vitro.Results. As a result of the study, 9 phenolic compounds were identified: rutin, quercetin, quercetin-3-b-glycoside, naringin, epicatechin, catechin, gallocatechin, epicatechin gallate. It was determined that rutin, quercetin, epicatechin, and catechin predominate in the leaves, shoots, and fruits of Virginia creeper.It was established that the extracts of leaves with shoots and fruits of Virginia creeper show a pronounced antioxidant activity due to their ability to inhibit autooxidation of adrenaline in vitro.Conclusions. The high content of rutin, quercetin, epicatechin and catechin in the leaves, shoots and fruits of Virginia creeper has scientific interest, due to discovered compounds have a valuable pharmacological effect. The obtained results can be used for the standardization of raw materials of Virginia creeper, and differences in the quantitative content of halocatechin in different types of raw materials, as well as the presence of naringin in leaves and shoots and luteolin in the fruits of Virginia creeper as diagnostic features of this species raw material.The results of the study of antioxidant activity confirm the prospects of using this raw material for the creation of new drugs with antioxidant activity
References
- Yu, J., Niu, Y., You, Y., Cox, C. J., Barrett, R. L., Trias‐Blasi, A. et al. (2022). Integrated phylogenomic analyses unveil reticulate evolution in Parthenocissus(Vitaceae), highlighting speciation dynamics in the Himalayan–Hengduan Mountains. New Phytologist, 238 (2), 888–903. doi: https://doi.org/10.1111/nph.18580
- Lu, L., Wen, J., Chen, Z. (2011). A combined morphological and molecular phylogenetic analysis of Parthenocissus (Vitaceae) and taxonomic implications. Botanical Journal of the Linnean Society, 168 (1), 43–63. doi: https://doi.org/10.1111/j.1095-8339.2011.01186.x
- Wen, J., Lu, L., Nie, Z., Liu, X., Zhang, N., Ickert‐Bond, S., Gerrath, J. et al. (2018). A new phylogenetic tribal classification of the grape family (Vitaceae). Journal of Systematics and Evolution, 56 (4), 262–272. doi: https://doi.org/10.1111/jse.12427
- Chen, Z. D., Ren, H., Wen, J.; Wu, Z. Y., Raven, P. H., Hong, D. Y. (Eds.) (2007). Parthenocissus Planchon. Flora of China. Vol. 12. Beijing – St. Louis: Science Press, Missouri Botanical Garden Press, 173–177.
- Mosyakin, S. L., Fedoronchuk M. F. (1999). Vascular plants of Ukraine. A nomenclatural checklist. Kyiv, 346.
- Ismail, N. R., Kadhim, E. J. (2021). Phytochemical Screening and Isolation of New Compounds. International Journal of Drug Delivery Technology, 11 (3), 1033–1039.
- Kovalenko, O. A., Kalista, M. S. (2019). Germination biology of Parthenocissus quinquefolia (L.) Planch. (Vitaceae). Thaiszia – journal of botany, 29 (2), 179–190. doi: https://doi.org/10.33542/tjb2019-2-04
- Pringle, J. S. (2010). Nomenclature of the thicket creeper. Parthenocissus inserta (Vitaceae). The Michigan Botanist, 49 (3), 73–78.
- Faisal, S., Perveen, A., Khan, Z.-U-D., Sardar, A. A., Shaheen, Sh., Manzoor, A. (2018). Phytochemical screening and antioxidant potential of Parthenocissus quinquefolia (L.) Planch extracts of bark and stem. Pakistan Journal of Pharmaceutical Sciences, 31 (5), 1813–1816.
- Liang, X., Gao, Y., Fei, W., Zou, Y., He, M., Yin, L. et al. (2018). Chemical characterization and antioxidant activities of polysaccharides isolated from the stems of Parthenocissus tricuspidata. International Journal of Biological Macromolecules, 119, 70–78. doi: https://doi.org/10.1016/j.ijbiomac.2018.07.131
- Kim, H. J., Saleem, M., Seo, S. H., Jin, C., Lee, Y. S. (2005). Two New Antioxidant Stilbene Dimers, Parthenostilbenins A and B fromParthenocissus tricuspidata. Planta Medica, 71 (10), 973–976. doi: https://doi.org/10.1055/s-2005-871229
- Kwack, M. H., Ha, D.-L., Lee, W. J. (2022). Preventative effects of antioxidants on changes in sebocytes, outer root sheath cells, and Cutibacterium acnes-pretreated mice by particulate matter: No significant difference among antioxidants. International Journal of Immunopathology and Pharmacology, 36. doi: https://doi.org/10.1177/03946320221112433
- Kumar, S., Kunaparaju, N., Zito, S. W., Barletta, M. A. (2011). Effect of Wrightia tinctoria and Parthenocissus quinquefolia on blood glucose and Insulin levels in the Zucker Diabetic Rat Model. Journal of Complementary and Integrative Medicine, 8 (1), 1–12. doi: https://doi.org/10.2202/1553-3840.1538
- Jang, M., Cai, L., Udeani, G. O., Slowing, K. V., Thomas, C. F., Beecher, C. W. W. et al. (1997). Cancer Chemopreventive Activity of Resveratrol, a Natural Product Derived from Grapes. Science, 275 (5297), 218–220. doi: https://doi.org/10.1126/science.275.5297.218
- Lamikanra, O., Grimm, C. C., Rodin, J. B., Inyang, I. D. (1996). Hydroxylated Stilbenes in Selected American Wines. Journal of Agricultural and Food Chemistry, 44 (4), 1111–1115. doi: https://doi.org/10.1021/jf950274j
- Lee, S. H., Liu, Q., Hwang, B. Y., Lee, M. K. (2013). Inhibitory Effects of Stilbene Derivatives from Parthenocissus Tricuspidata on Adipocyte Differentiation and Pancreatic Lipase. Natural Product Communications, 8 (10), 1439–1441. doi: https://doi.org/10.1177/1934578x1300801026
- Tanaka, T., Ohyama, M., Morimoto, K., Asai, F., Iinuma, M. (1998). A resveratrol dimer from Parthenocissus tricuspidata. Phytochemistry, 48 (7), 1241–1243. doi: https://doi.org/10.1016/s0031-9422(97)00898-4
- He, S., Lu, Y., Wu, B., Pan, Y. (2007). Isolation and purification of antioxidative isomeric polyphenols from the roots of Parthenocissus laetevirens by counter-current chromatography. Journal of Chromatography A, 1151 (1-2), 175–179. doi: https://doi.org/10.1016/j.chroma.2007.02.102
- Jeon, J.-S., Kim, C. Y. (2013). Preparative separation and purification of flavonoids and stilbenoids from Parthenocissus tricuspidata stems by dual-mode centrifugal partition chromatography. Separation and Purification Technology, 105, 1–7. doi: https://doi.org/10.1016/j.seppur.2012.11.010
- Hwang, H. K., Sung, H. K., Wang, W. K., Kim, I. H. (1995). Flavonol glycosides from Parthenocissus tricuspidata. Yakhak Hoechi, 39 (3), 289–296.
- Saleem, M., Kim, H. J., Jin, C., Lee, Y. S. (2004). Antioxidant caffeic acid derivatives from leaves ofparthenocissus tricuspidata. Archives of Pharmacal Research, 27 (3), 300–304. doi: https://doi.org/10.1007/bf02980064
- Kuznietsova, V. Yu., Kyslychenko, V. S., Adamenko, K. V. (2007). Analysis of lipophilic fractions of wild grape leaves. Pharmaceutical Review, 2, 82–85.
- Nguyen, P.-H., Zhao, B. T., Lee, J. H., Kim, Y. H., Min, B. S., Woo, M. H. (2014). Antithrombotic Phenolics from the Stems of Parthenocissus tricuspidata Possess Anti-inflammatory Effect. Bulletin of the Korean Chemical Society, 35 (6), 1763–1768. doi: https://doi.org/10.5012/bkcs.2014.35.6.1763
- Konovalova, O. Yu., Yashchuk, B. O., Hurtovenko, I. O. (2021). Vyznachennia vmistu vilnykh mono- ta dysakharydiv u syrovyni divochoho vynohradu piatylystochkovoho. Ideas and innovations in natural sciences. Lublin, 154–156. doi: https://doi.org/10.30525/978-9934-26-047-6-41
- Yang, J., Wang A., Ji, T., Su, Y. (2010). Chemical constituents from Parthenocissus quinquefolia. China Journal of Chinese Materia Medica, 35 (12), 1573–1576. doi: https://doi.org/10.4268/cjcmm20101215
- Khan, Z. U. D., Faisal, S., Perveen, A., Sardar, A. A., Siddiqui, S. Z. (2018). Phytochemical properties and antioxidant activities of leaves and fruits extracts of Parthenocissus quinquefolia (L.) Planch. Bangladesh Journal of Botany, 47 (1), 33–38.
- Gai, C.Y., Liu, H.M., Li, J., Li, J. (2010). Extraction and Content Determination of Total Flavonoid in Parthenocissus quinquefolia. Special Wild Economic Animal and Plant Research, 2, 20.
- Shi, J., Han, X., Zhang, Y., Sun, T., Diao, H., Cao, X. (2010). Extraction techniques and identification of flavonoids in parthenocissus seeds. China Med. Her. 18, 33.
- Zhao, X., Zhang, Y., Long, T., Wang, S., Yang, J. (2022). Regulation Mechanism of Plant Pigments Biosynthesis: Anthocyanins, Carotenoids, and Betalains. Metabolites, 12 (9), 871. doi: https://doi.org/10.3390/metabo12090871
- Ben Ticha, M., Meksi, N., Attia, H. E., Haddar, W., Guesmi, A., Ben Jannet, H., Mhenni, M. F. (2017). Ultrasonic extraction of Parthenocissus quinquefolia colorants: Extract identification by HPLC-MS analysis and cleaner application on the phytodyeing of natural fibres. Dyes and Pigments, 141, 103–111. doi: https://doi.org/10.1016/j.dyepig.2017.02.002
- Mathew, S., Abraham, T. E., Zakaria, Z. A. (2015). Reactivity of phenolic compounds towards free radicals under in vitro conditions. Journal of Food Science and Technology, 52(9), 5790–5798. doi: https://doi.org/10.1007/s13197-014-1704-0
- Mohamed, A. A., Salah, M. M., El-Dein, M. M. Z., EL-Hefny, M., Ali, H. M., Farraj, D. A. A. et al. (2021). Ecofriendly Bioagents, Parthenocissus quinquefolia, and Plectranthus neochilus Extracts to Control the Early Blight Pathogen (Alternaria solani) in Tomato. Agronomy, 11 (5), 911. doi: https://doi.org/10.3390/agronomy11050911
- Batiha, G. E.-S., Beshbishy, A. M., Ikram, M., Mulla, Z. S., El-Hack, M. E. A., Taha, A. E. et al. (2020). The Pharmacological Activity, Biochemical Properties, and Pharmacokinetics of the Major Natural Polyphenolic Flavonoid: Quercetin. Foods, 9 (3), 374. doi: https://doi.org/10.3390/foods9030374
- Ullah, A., Munir, S., Badshah, S. L., Khan, N., Ghani, L., Poulson, B. G. et al. (2020). Important Flavonoids and Their Role as a Therapeutic Agent. Molecules, 25 (22), 5243. doi: https://doi.org/10.3390/molecules25225243
- Budniak, L., Vasenda, M., Marchyshyn, S., Kurylo, K. (2020). Determination of the optimum extraction regime of reducing compounds and flavonoids of Primula denticulata Smith leaves by a dispersion analysis. Pharmacia, 67 (4), 373–378. doi: https://doi.org/10.3897/pharmacia.67.e54170
- Pyrzynska, K., Sentkowska, A. (2019). Chromatographic analysis of polyphenols. Polyphenols in plants. Academic Press, 353–364. doi: https://doi.org/10.1016/b978-0-12-813768-0.00021-9
- Tao, W., Zhou, Z., Zhao, B., Wei, T. (2016). Simultaneous determination of eight catechins and four theaflavins in green, black and oolong tea using new HPLC–MS–MS method. Journal of Pharmaceutical and Biomedical Analysis, 131, 140–145. doi: https://doi.org/10.1016/j.jpba.2016.08.020
- Hurtovenko, I. O. (2020). Porivnialne farmakohnostychne doslidzhennia deiakykh vydiv rodu ahastakhe (Agastache J.Clayton ex Gronov). Kyiv: ZDMU, 267.
- Enogieru, A. B., Haylett, W., Hiss, D. C., Bardien, S., Ekpo, O. E. (2018). Rutin as a Potent Antioxidant: Implications for Neurodegenerative Disorders. Oxidative Medicine and Cellular Longevity, 2018, 1–17. doi: https://doi.org/10.1155/2018/6241017
- Oyagbemi, A. A., Bolaji‐Alabi, F. B., Ajibade, T. O., Adejumobi, O. A., Ajani, O. S., Jarikre, T. A. et al. (2020). Novel antihypertensive action of rutin is mediated via inhibition of angiotensin converting enzyme/mineralocorticoid receptor/angiotensin 2 type 1 receptor (ATR1) signaling pathways in uninephrectomized hypertensive rats. Journal of Food Biochemistry, 44 (12). doi: https://doi.org/10.1111/jfbc.13534
- Zupanets, I. A., Holubovska, O. A., Shkurba, A. V., Shebeko, S. K., Shalamai, A. S. (2020). Perspektyvy vyvchennia zastosuvannia preparativ kvertsetynu v likuvanni COVID-19. Ukrainian Medical Journal, 136 (1), 75–78. doi: https://doi.org/10.32471/umj.1680-3051.136.177136
- Yang, D., Wang, T., Long, M., Li, P. (2020). Quercetin: Its Main Pharmacological Activity and Potential Application in Clinical Medicine. Oxidative Medicine and Cellular Longevity, 2020, 1–13. doi: https://doi.org/10.1155/2020/8825387
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Olena Konovalova, Bohdana Yashchuk, Iryna Hurtovenko, Olha Shcherbakova, Mariia Kalista, Natalia Sydora

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






