Development of the spectrophotometric method for the determination of meldonium in capsules by using alizarine
DOI:
https://doi.org/10.15587/2519-4852.2024.299165Keywords:
alizarin, meldonium, spectrophotometry, validation, quantitative determination, capsulesAbstract
The aim of the work was to develop a simple, rapid, economic spectrophotometric method for the determination of meldonium in capsules based on the reaction with alizarin.
Materials and methods. Analytical equipment: double-beam UV-visible spectrophotometer Shimadzu UV 1800 (Japan), a pair of 1 cm matched quartz cells, software UV-Probe 2.62, laboratory electronic balance RAD WAG AS 200/C, pH-meter И-160МИ. Pharmacopoeial standard sample (CRS) of meldonium dihydrate (Sigma-Aldrich, (≥ 98 %, HPLC)), alizarin (Synbias), capsules Metamax (Darnytsia) 250 mg, Vasopro (Farmak) 500 mg, Mildronate (Grindex) 500 mg, dimethylformamide (“Honeywell Riedel-de Haen”).
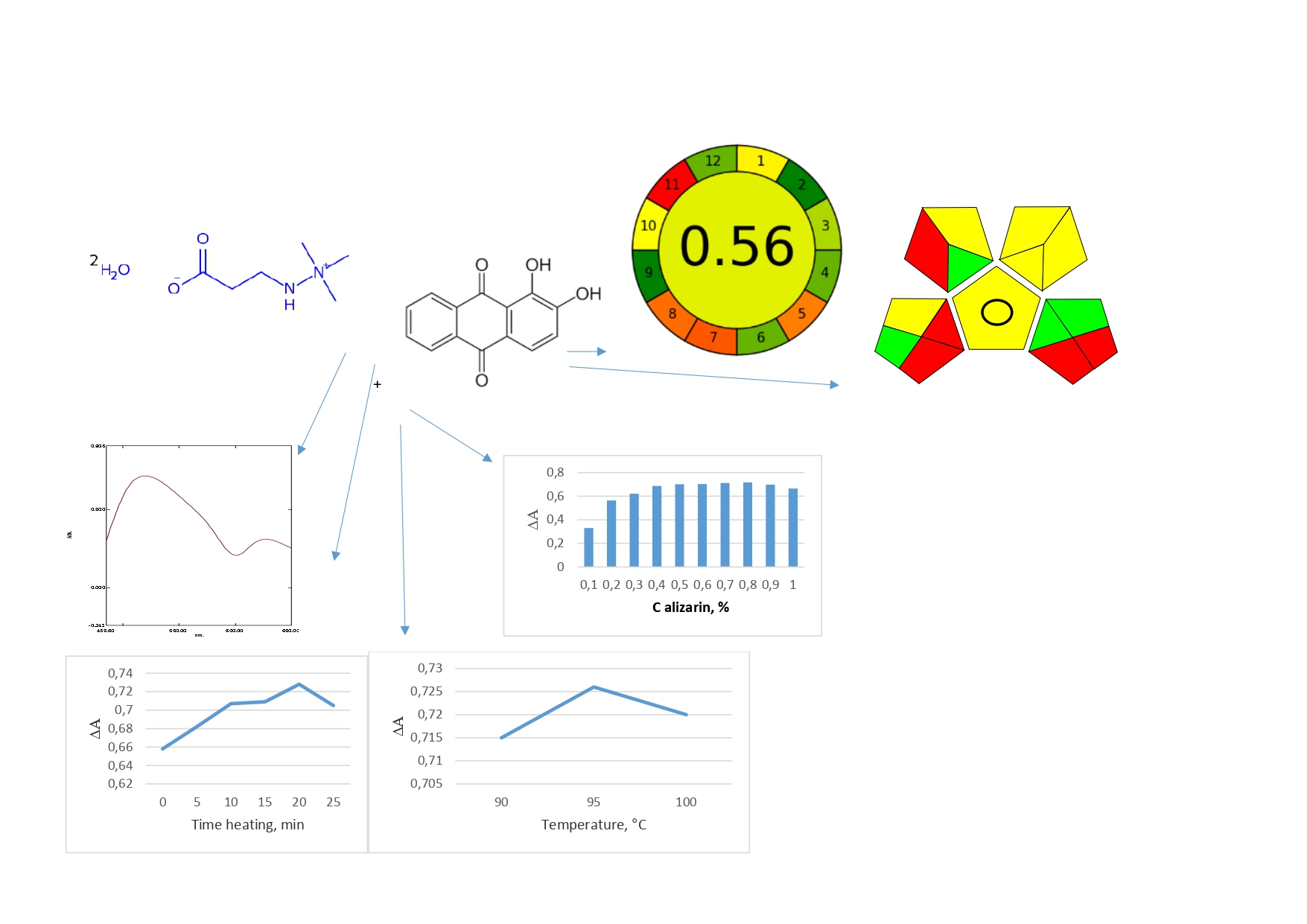
Results and discussion. A spectrophotometric method for determining meldonium in capsules by reaction with alizarine has been developed. The absorption maximum of the formed complex in dimethylformamide was at a wavelength of 517 nm. Stoichiometric ratios of reactive components «meldonium- alizarin» were 1:1. Validation of the developed analytical method for the determination of meldonium in medicines was carried out in accordance with the requirements of the SPhU. The optimal conditions for performing the quantitative determination of meldonium have been established: concentration of alizarin solution – 0.8 %, volume 0.8 % alizarin solution – 0.5 ml, heating time – 20 min, temperature – 95+/- 2 °C. Linearity has been in the concentration range of 0.0402- 0.1073 mg/mL, the limit of detection - 2.84 μg/mL, and the limit of quantification – 8.59 μg/mL. The eco-friendliness of the developed analytical method was carried out using the analytical eco-scale, AGREE, and GAPI methods.
Conclusions. The developed method can be used as an arbitration method for the routine analysis of meldonium capsules
References
- Meldonium. Available at: https://go.drugbank.com/drugs/DB13723
- Dambrova, M. (2002). Mildronate Cardioprotective Action through Carnitine-Lowering Effect. Trends in Cardiovascular Medicine, 12 (6), 275–279. https://doi.org/10.1016/s1050-1738(02)00175-5
- Sjakste, N., Kalvinsh, I. (2006). Mildronate: an antiischemic drug with multiple indications. Pharmacologyonline, 1, 1–18.
- Volynskyi, D., Vakaliuk, I. (2019). Use of meldonium in the treatment of patients with coronary artery disease and concomitant arterial hypertension. EUREKA: Health Sciences, 6, 9–14. https://doi.org/10.21303/2504-5679.2019.001018
- European Pharmacopoeia. 11 ed. (2021). Available at: https://www.edqm.eu/en/european-pharmacopoeia-ph.-eur.-11th-edition
- Donchenko, А., Nahorna, N., Vasyuk, S. (2018). Development and validation of spectrophotometric method for the determination of meldonium dihydrate in dosage forms. ScienceRise: Pharmaceutical Science, 4 (14), 23–27. https://doi.org/10.15587/2519-4852.2018.141397
- Pidpruzhnykov, Y. V., Sabko, V. E., Iurchenko, V. V., Zupanets, I. A. (2011). UPLC‐MS/MS method for bioequivalence study of oral drugs of meldonium. Biomedical Chromatography, 26 (5), 599–605. https://doi.org/10.1002/bmc.1703
- Lv, Y.-F., Hu, X., Bi, K.-S. (2007). Determination of mildronate in human plasma and urine by liquid chromatography–tandem mass spectrometry. Journal of Chromatography B, 852 (1-2), 35–39. https://doi.org/10.1016/j.jchromb.2006.12.031
- Peng, Y., Yang, J., Wang, Z., Wang, J., Liu, Y., Luo, Z., Wen, A. (2010). Determination of mildronate by LC–MS/MS and its application to a pharmacokinetic study in healthy Chinese volunteers. Journal of Chromatography B, 878 (5-6), 551–556. https://doi.org/10.1016/j.jchromb.2009.12.030
- Görgens, C., Guddat, S., Dib, J., Geyer, H., Schänzer, W., Thevis, M. (2015). Mildronate (Meldonium) in professional sports – monitoring doping control urine samples using hydrophilic interaction liquid chromatography – high resolution/high accuracy mass spectrometry. Drug Testing and Analysis, 7 (11-12), 973–979. Portico. https://doi.org/10.1002/dta.1788
- Horyn, M., Logoyda, L. (2020). Bioanalytical method development and validation for the determination of metoprolol and meldonium in human plasma. Pharmacia, 67 (2), 39–48. https://doi.org/10.3897/pharmacia.67.e50397
- Oliveira, D., de Araújo, A., Ribeiro, W., Silva, D., Duarte, A. C., de Sousa, V., Pereira, H. G. (2021). Screening method of mildronate and over 300 doping agents by reversed-phase liquid chromatography-high resolution mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis, 195, 113870. https://doi.org/10.1016/j.jpba.2020.113870
- Kim, Y., Jeong, D., Min, H., Sung, C., Park, J. H., Son, J., Kim, K. H. (2017). Method for screening and confirming meldoni- um in human urine by high-resolution mass spectrometry and identification of endogenous interferences for anti-doping testing. Mass Spectrometry Letters, 8 (2), 39–43. https://doi.org/10.5478/MSL.2017.8.2.39
- Parr, M. K., & Botrè, F. (2022). Supercritical fluid chromatography mass spectrometry as an emerging technique in doping control analysis. TrAC Trends in Analytical Chemistry, 147, 116517. https://doi.org/10.1016/j.trac.2021.116517
- Görgens, C., Guddat, S., Bosse, C., Geyer, H., Pop, V., Schänzer, W., Thevis, M. (2017). The atypical excretion profile of meldonium: Comparison of urinary detection windows after single- and multiple-dose application in healthy volunteers. Journal of Pharmaceutical and Biomedical Analysis, 138, 175–179. https://doi.org/10.1016/j.jpba.2017.02.011
- Cai, L.-J., Zhang, J., Peng, W.-X., Zhu, R.-H., Yang, J., Cheng, G., Wang, X.-M. (2011). Determination of Mildronate in Human Plasma and Urine by UPLC–Positive Ion Electrospray Tandem Mass Spectrometry. Chromatographia, 73 (7-8), 659–665. https://doi.org/10.1007/s10337-010-1839-8
- Tretzel, L., Görgens, C., Geyer, H., Thomas, A., Dib, J., Guddat, S. et al. (2016). Analyses of Meldonium (Mildronate) from Blood, Dried Blood Spots (DBS), and Urine Suggest Drug Incorporation into Erythrocytes. International Journal of Sports Medicine, 37 (6), 500–502. https://doi.org/10.1055/s-0036-1582317
- Rabin, O., Uiba, V., Miroshnikova, Y., Zabelin, M., Samoylov, A., Karkischenko, V. et al. (2018). Meldonium long‐term excretion period and pharmacokinetics in blood and urine of healthy athlete volunteers. Drug Testing and Analysis, 11 (4), 554–566. https://doi.org/10.1002/dta.2521
- Forsdahl, G., Jančić-Stojanović, B., Anđelković, M., Dikić, N., Geisendorfer, T., Jeitler, V., Gmeiner, G. (2018). Urinary excretion studies of meldonium after multidose parenteral application. Journal of Pharmaceutical and Biomedical Analysis, 161, 289–295. https://doi.org/10.1016/j.jpba.2018.08.053
- Rusu, L. D., Bratu, I., Măruțoiu, C., Moldovan, Z., Rada, M. (2020). Analytical methods for meldonium determination in urine samples. Analytical Letters, 54 (1-2), 233–241. https://doi.org/10.1080/00032719.2020.1748043
- Temerdashev, A., Azaryan, A., Dmitrieva, E. (2020). Meldonium determination in milk and meat through UHPLC-HRMS. Heliyon, 6 (8), e04771. https://doi.org/10.1016/j.heliyon.2020.e04771
- Alizarin. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/Alizarin Last accessed: 10.05.2023
- State Pharmacopoeia of Ukraine. Vol. 1 (2015). Kharkiv: SE “Ukrainian Scientific Pharmacopoeial Center for Quality of Medicines, 11148.
- Gałuszka, A., Migaszewski, Z. M., Konieczka, P., Namieśnik, J. (2012). Analytical Eco-Scale for assessing the greenness of analytical procedures. TrAC Trends in Analytical Chemistry, 37, 61–72. https://doi.org/10.1016/j.trac.2012.03.013
- Pena-Pereira, F., Wojnowski, W., Tobiszewski, M. (2020). AGREE –Analytical GREEnness Metric Approach and Software. Analytical Chemistry, 92 (14), 10076–10082. https://doi.org/10.1021/acs.analchem.0c01887
- Płotka-Wasylka, J. (2018). A new tool for the evaluation of the analytical procedure: Green Analytical Procedure Index. Talanta, 181, 204–209. https://doi.org/10.1016/j.talanta.2018.01.013
- Płotka-Wasylka, J., Wojnowski, W. (2021). Complementary green analytical procedure index (ComplexGAPI) and software. Green Chemistry, 23 (21), 8657–8665. https://doi.org/10.1039/d1gc02318g
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Mariana Horyn, Marjan Piponski, Olha Poliak, Nataliia Shulyak, Marta Sulyma, Liliya Logoyda

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






