Determination of chromatographic conditions for quantitative assessment of active components in complex nasal spray after manufacturing and expiry date
DOI:
https://doi.org/10.15587/2519-4852.2024.299184Keywords:
spray, phenylephrine hydrochloride, lidocaine hydrochloride, polyvinylpyrrolidone, panthenol, nitrofural, diphenhydramine hydrochloride, quantitative determination, liquid chromatography, stabilityAbstract
The aim of the work is the development of chromatographic conditions, the study of the validation characteristics of the method of quantitative determination of phenylephrine hydrochloride, nitrofural, lidocaine hydrochloride and diphenhydramine hydrochloride, panthenol, povidone in the joint presence in the nasal spray by a complex method of liquid chromatography with UV detection. Evaluation of the quantitative content of active components after manufacturing and during the shelf life.
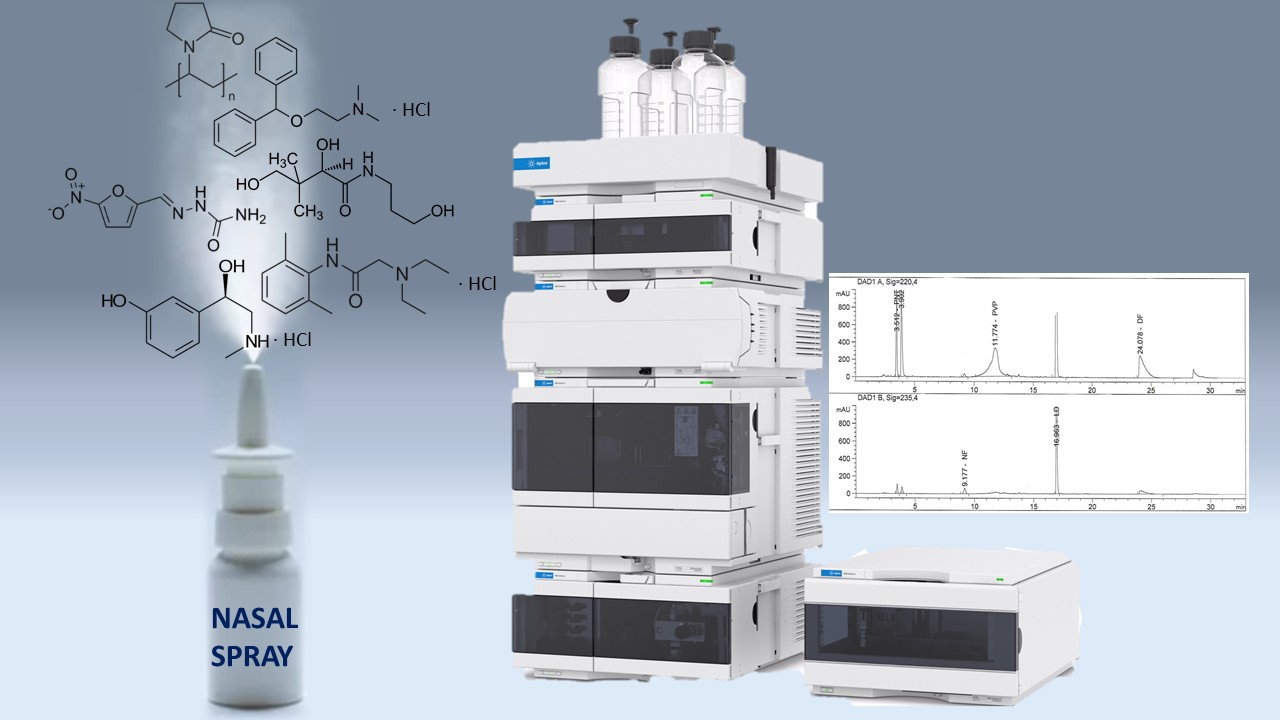
Materials and methods. Agilent 1260 liquid chromatographs, equipped with a diode-matrix detector from the company "Agilent technologies", USA. Chromatographic columns 250×4.6 mm in size, filled with octadecylsilyl silica gel for chromatography (Zorbax StableBond SB-Aq, Agilent company), mobile phase A - phosphate buffer solution pH 7.0 - acetonitrile P (1650:350), mobile phase B – acetonitrile P; elution mode – gradient; mobile phase flow rate – 1.0 ml/min; detection wavelengths – 220 nm (for panthenol, phenylephrine, povidone, diphenhydramine) and 235 nm (for nitrofural and lidocaine).
Results. Chromatographic separation conditions were developed for the co-presence determination of six target substances: panthenol, phenylephrine hydrochloride, nitrofural, povidone, lidocaine hydrochloride and diphenhydramine hydrochloride. The suitability of the technique for this task was confirmed by determining the validation characteristics. The methodology at the appropriate level is characterized by specificity, linearity, correctness and convergence in the range of application for panthenol (range 20.33-38.26 mg/ml, ΔZ=0.93 ≤ max ΔZ=3.20, a=0.63 max a=5.12, r = 0.9978 min r= 0.9924), phenylephrine hydrochloride (range 1,70-3,21 mg/ml, ΔZ=0.51 ≤ max ΔZ=3.20, a=0.15 max a=5.12, r = 0.9984 min r= 0.9924), nitrofural (range 0.137-0.257 mg/ml, ΔZ=0.91 ≤ max ΔZ=3.20, a=0.032 max a=5.12, r = 0.9987 min r= 0.9924) povidone (range 20,44-38,50 mg/ml, ΔZ=0.23 ≤ max ΔZ=3.20, a=2,33 max a=5.12, r = 0.9942 min r= 0.9924), lidocaine hydrochloride (range 6,80-12,81 mg/ml, ΔZ=0.34 ≤ max ΔZ=3.20, a=0.66 max a=5.12, r = 0.9988 min r= 0.9924), diphenhydramine hydrochloride (range 1,36-2,56 mg/ml, ΔZ=0.20 ≤ max ΔZ=3.20, a=0.15 max a=5.12, r = 0.9980 min r= 0.9924). There are no significant changes when stored at 25 °C for 6 months.
Conclusions. An analytical method of quantitative determination of the component composition in an extemporaneous nasal spray by a complex method of high-performance liquid chromatography has been developed. The determined validation parameters confirm the correctness of the methodology. The chemical stability of the dosage form is observed for 6 months
References
- Pro zatverdzhennia Unifikovanoho klinichnoho protokolu pervynnoi ta spetsializovanoi medychnoi dopomohy "Hostryi rynosynusyt" (2023). Nakaz MOZ Ukrainy No. 1793.13.10.2023. Available at: https://moz.gov.ua/article/ministry-mandates/nakaz-moz-ukraini-vid-13102023--1793-pro-zatverdzhennja-unifikovanogo-klinichnogo-protokolu-pervinnoi-ta-specializovanoi-medichnoi-dopomogi-gostrij-rinosinusit-unifikovanogo-klinichnogo-protokolu-pervinnoi-ta-specializovanoi-medichnoi
- Savchenko, L. P., Georgiyants, V. A. (2020). Current trends in compounding of medicines and its legislative regulation in foreign countries. Farmatsevtychnyi Zhurnal, 4 (75), 6–17. https://doi.org/10.32352/0367-3057.4.20.01
- Samborskyi, O. S. (2018). Investigation of the opportunities of manufacturing pharmacy in ukraine and abroad. Pharmaceutical Review, (1), 102–114. https://doi.org/10.11603/2312-0967.2018.1.8695
- Cherniakova, V., Bevz, N., Strilets, O., Harna, N., Bevz, O., Yevtifieieva, O. (2023). The study of the stability of silver proteinate solutions prepared in pharmacies. ScienceRise: Pharmaceutical Science, 5 (45), 24–31. https://doi.org/10.15587/2519-4852.2023.289798
- Al-Salman, H. N. K., Al-Jadaan Shaker, A. N., Alnuaim, M., Hussein, H. H. (2017). Estimation of Lidocaine-HCl in Pharmaceutical drugs by HPLC-UV System. American Journal of PharmTech Research, 7 (1), 369–378.
- Diphenhydramine hydrochloride oral solution, USP43. Available at: https://www.uspnf.com/ Last accessed: 27.05.2023
- Diphenhydramine hydrochloride. 01/2016:0023, Ph.Eur. 11.0 (2016). Available at: https://pheur.edqm.eu/home Last accessed: 27.05.2023
- Derzhavna Farmakopeia Ukrainy. Vol. 3. Kharkiv: Derzhavne pidpryiemstvo «Ukrainskyi naukovyi farmakopeinyi tsentr yakosti likarskykh zasobiv», 732.
- Phenylephrine hydrochloride, USP43. Available at: https://www.uspnf.com/ Last accessed: 21.05.2023
- Nitrofural, 01/2022:1135, Ph.Eur., 11.0. Available at: https://pheur.edqm.eu/home Last accessed: 27.05.2023
- Mahboubi, A., Gholamreza Alviri, M., Afshar, M., Farhangi, M. (2019). Development and Validation of A Fast, Simple And Specific Stability Indicating RP-HPLC Method for Determination of Dexpanthenol in Eye Gel Formulation. Iranian Journal of Pharmaceutical Research, 18 (2), 670–676. https://doi.org/10.22037/ijpr.2019.1100681
- Weiss, C. L., Fairchild, M. R., Stanton, B., Nshime, B. S., Parkanzky, P. D. (2019). Innovative Method for the Analysis of Dexpanthenol in Hair Care Products. Journal of AOAC INTERNATIONAL, 102 (2), 633–637. https://doi.org/10.5740/jaoacint.18-0053
- Povidone, USP43-NF38, 3631. Available at: https://www.uspnf.com/ Last accessed: 05.06.2023
- Povidone, 04/2024:0685, Ph.Eur., 11.4. Available at: https://pheur.edqm.eu/home Last accessed: 27.08.2023
- Kirkpatrick, D., Fain, M., Yang, J., Santos, L., Anthony, C. (2019). UHPLC assay and impurity methods for diphenhydramine and phenylephrine hydrochloride oral solution. SEPARATION SCIENCE PLUS, 3 (1-2), 4–11. https://doi.org/10.1002/sscp.201900084
- Palur, K., Archakam, S. C., Koganti, B. (2020). Chemometric assisted UV spectrophotometric and RP-HPLC methods for simultaneous determination of paracetamol, diphenhydramine, caffeine and phenylephrine in tablet dosage form. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 243, 118801. https://doi.org/10.1016/j.saa.2020.118801
- Cherniakova, V., Myhal, A., Rudiuk, V., Studenyak, Y., Kryvanych, O., Bevz, N., Georgiyants, V. (2023). Design and validation of analytical methods for quantitative determination of active ingredients in extemporal combined medicine in spray form. ScienceRise: Pharmaceutical Science, 6 (46), 31–40. https://doi.org/10.15587/2519-4852.2023.294919
- KNOWLEDGE Database. Available at: https://www.edqm.eu/en/knowledge-database Last accessed: 27.05.2023
- Derzhavna farmakopeia Ukrainy. Dopovnennia 4 (2.4) (2020). Kharkiv: Derzhavne pidpryiemstvo «Ukrainskyi naukovyi farmakopeinyi tsentr yakosti likarskykh zasobiv», 600.
- Derzhavna Farmakopeia Ukrainy. Vol. 1. Kharkiv: Derzhavne pidpryiemstvo «Ukrainskyi naukovyi farmakopeinyi tsentr yakosti likarskykh zasobiv», 1028.
- ICH guideline Q2(R2) on validation of analytical procedures Step 2b (2022). European Medicines Agency, 39.
- Yevtifieieva, O. A., Georgiyants, V. A. (2021). Medicines manufactured in pharmacies: features of validation of analytical methods and tests (Prior to the introduction of the monograph section of the SPU). Farmatsevtychnyi Zhurnal, 6, 80–93. https://doi.org/10.32352/0367-3057.6.21.08
- ICH Q1A (R2) Stability testing of new drug substances and drug products Step 5 (2003). European Medicines Agency, 20.
- ST-N MOZU 42-3.3:2004 Likarski zasoby. Nastanova z yakosti. Vyprobuvannia stabilnosti (2012). Kyiv: Ministerstvo okhorony zdorovia Ukrainy. Available at: https://compendium.com.ua/uk/clinical-guidelines-uk/standartizatsiya-farmatsevtichnoyi-produktsiyi-tom-1/st-n-mozu-42-3-3-2004/
- Lidocaine, USP43-NF38, 2620. Available at: https://www.uspnf.com/ Last accessed: 05.06.2023
- Lidocaine hydrochloride monohydrate, 01/2019:0227, Ph.Eur., 11.4. Available at: https://pheur.edqm.eu/home Last accessed: 27.07.2023
- Lidocaine hydrochloride, USP43-NF38, 2623. Available at: https://www.uspnf.com/ Last accessed: 05.06.2023)
- <115> Dexpanthenol assay, USP43-NF38, 6547. Available at: https://www.uspnf.com/ Last accessed: 05.06.2023
- Dexpanthenol, 04/2023:0761, Ph.Eur., 11.4 Available at: https://pheur.edqm.eu/home Last accessed: 27.07.2023
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Valeriia Cherniakova, Artem Myhal, Vitalii Rudiuk, Oleksandr Kryvanych, Olha Rudakova, Igor Tugaibei, Nataliia Bevz, Victoriya Georgiyants

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






