Dichloroacetic acid derivatives as potential anti-tumor and anti-inflammatory agents
DOI:
https://doi.org/10.15587/2519-4852.2024.299229Keywords:
dichloroacetate, dichloroacetic acid, dichloroacetamide, hybrid molecules, antitumor activity, anti-inflammatory activity, cholesterol, tumors, apoptosis, chemotherapyAbstract
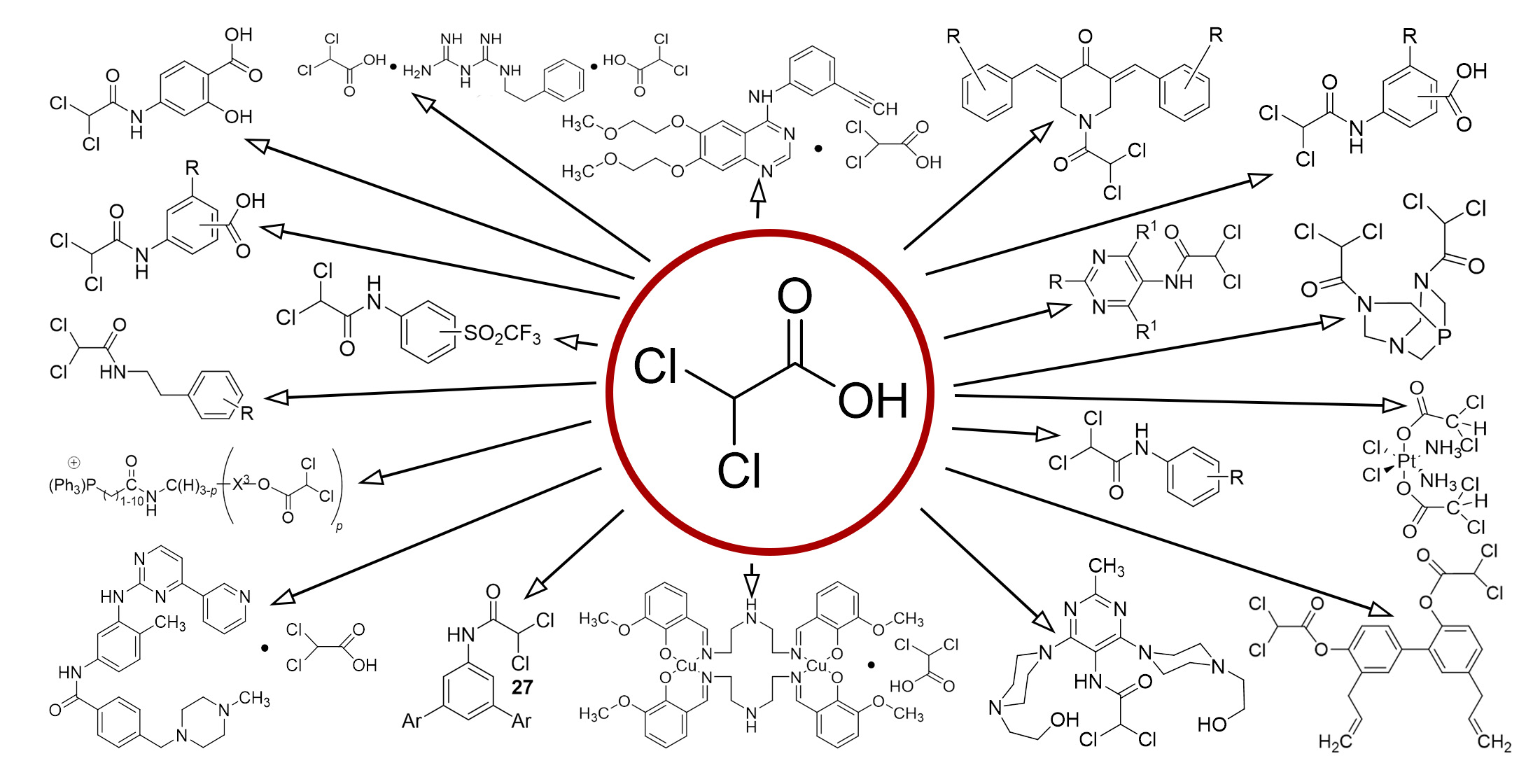
The aim. This review aims to provide a comprehensive understanding of dichloroacetic acid derivatives. We aim to cover all aspects of these compounds, including their chemical properties, various synthesis methods, and their wide range of applications in medicinal chemistry. By exploring their diverse roles in drug development, we aim to highlight their importance and potential in shaping future pharmaceutical innovation.
Materials and methods. Bibliosemantic and analytical methods are used in the research.
Results. Our studies confirm the potential effectiveness of dichloroacetic acid and its derivatives in the treatment of cancer and other diseases. These compounds can induce the apoptosis process, which is the programmed cell death, and inhibit the cancer cells' growth. This is particularly effective when dichloroacetic acid and its derivatives are used in combination with other therapeutic methods, as indicated in the patents cited in our study. Dichloroacetic acid and its derivatives have also shown the ability to lower blood glucose and cholesterol levels. This indicates the possibility of their use for diabetes, hyperlipidemia, and lactic acidosis treatment. Diabetes, hyperlipidemia, and lactic acidosis are serious conditions that can lead to significant health problems. Therefore, the possibility of using dichloroacetic acid and its derivatives for the treatment of these conditions opens new perspectives in medical science.
Conclusions. Our findings point to the prospects of further research in the field of new therapy methods development and the use of dichloroacetic acid derivatives as potential drugs to improve the effectiveness of cancer and other diseases treatment. We believe that these compounds have great potential for further study and may play an important role in future medical innovation
References
- Singh, Y., Singh, R. (2008). Theoretical studies of different tautomers of anti-cancer drug: dichloroacetate. Pakistan Journal of Pharmaceutical Sciences, 21 (4), 390–395.
- Dichloressigsäure, Natriumdichloracetat. Kurzfassung toxikologische bewertungen. Nr. 188 b Dichloressigsäure, Natriumdichloracetat 03/06, BG-Chemie.
- Tao, L., Kerou, W., Jie, C., Bingliang, Z., Li, Z., Dan, C. et al. (2018). Pat. No. CN108658756A. Method for preparing dichloroacetic acid by selective dechlorination of trichloroacetic acid. Xian Catalyst New Mat CO LTD (China); published: 16.10.2018.
- Wheeler, A. S., Smith, S. C. (1923). Direct conversion of derivatives of dichloro-acetic acid into derivatives of trichloro-acetic acid. Journal of the American Chemical Society, 45 (8), 1994–1998. https://doi.org/10.1021/ja01661a021
- Katon, J. E., Stout, T. H., Hess, G. G. (1986). The Infrared Spectra of Dichloroacetic Acid Derivatives: Characteristic Absorption Frequencies of the -CHCl2 Group. Applied Spectroscopy, 40 (1), 1–3. https://doi.org/10.1366/0003702864815501
- Antonius, C., Joannes, M. (1985). Pat. No. CA1189867A. Preparation of derivatives of dichloroacetic acid esters. Stamicarbon (NL); published 02.07.1985.
- Darensbourg, D. J., Ortiz, C. G., Kamplain, J. W. (2004). A New Water-Soluble Phosphine Derived from 1,3,5-Triaza-7-phosphaadamantane (PTA), 3,7-Diacetyl-1,3,7-triaza-5-phosphabicyclo[3.3.1]nonane. Structural, Bonding, and Solubility Properties. Organometallics, 23 (8), 1747–1754. https://doi.org/10.1021/om0343059
- Krogstad, D. A., Ellis, G. S., Gunderson, A. K., Hammrich, A. J., Rudolf, J. W., Halfen, J. A. (2007). Two new water-soluble derivatives of 1,3,5-triaza-7-phosphaadamantane (PTA): Synthesis, characterization, X-ray analysis and solubility studies of 3,7-diformyl-1,3,7-triaza-5-phosphabicyclo[3.3.1]nonane and 1-pyridylmethyl-3,5-diaza-1-azonia-7-phosphatricyclo[3.3.1.1]decane bromide. Polyhedron, 26 (15), 4093–4100. https://doi.org/10.1016/j.poly.2007.05.003
- Darensbourg, D. J., Robertson, J. B., Larkins, D. L., Reibenspies, J. H. (1999). Water-Soluble Organometallic Compounds. 7.1Further Studies of 1,3,5-Triaza-7-Phosphaadamantane Derivatives of Group 10 Metals, Including Metal Carbonyls and Hydrides. Inorganic Chemistry, 38 (10), 2473–2481. https://doi.org/10.1021/ic981243j
- Guerrero, E., Miranda, S., Lüttenberg, S., Fröhlich, N., Koenen, J.-M., Mohr, F. et al. (2013). trans-Thionate Derivatives of Pt(II) and Pd(II) with Water-Soluble Phosphane PTA and DAPTA Ligands: Antiproliferative Activity against Human Ovarian Cancer Cell Lines. Inorganic Chemistry, 52 (11), 6635–6647. https://doi.org/10.1021/ic4006746
- Carreira, M., Calvo-Sanjuán, R., Sanaú, M., Marzo, I., Contel, M. (2012). Organometallic Palladium Complexes with a Water-Soluble Iminophosphorane Ligand As Potential Anticancer Agents. Organometallics, 31 (16), 5772–5781. https://doi.org/10.1021/om3006239
- Marvelli, L., Ferretti, V., Bertolasi, V., Lampronti, I., Gambari, R., Trapella, C. et al. (2019). A new amido-phosphine of dichloroacetic acid as an active ligand for metals of pharmaceutical interest. Synthesis, characterization and tests of antiproliferative and pro-apoptotic activity. Journal of Inorganic Biochemistry, 199, 110787. https://doi.org/10.1016/j.jinorgbio.2019.110787
- Arjunan, V., Mohan, S., Subramanian, S., Thimme Gowda, B. (2004). Synthesis, Fourier transform infrared and Raman spectra, assignments and analysis of N-(phenyl)- and N-(chloro substituted phenyl)-2,2-dichloroacetamides. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 60 (5), 1141–1159. https://doi.org/10.1016/j.saa.2003.07.003
- Arjunan, V., Senthilkumari, S., Ravindran, P., Mohan, S. (2014). Synthesis, FTIR and FT-Raman spectral analysis and structure–activity relations of N-(4-bromophenyl)-2,2-dichloroacetamide by DFT studies. Journal of Molecular Structure, 1064, 15–26. https://doi.org/10.1016/j.molstruc.2014.01.091
- Arjunan, V., Ravindran, P., Subhalakshmi, K., Mohan, S. (2009). Synthesis, structural, vibrational and quantum chemical investigations of N-(2-methylphenyl)-2,2-dichloroacetamide and N-(4-methylphenyl)-2,2-dichloroacetamide. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 74 (3), 607–616. https://doi.org/10.1016/j.saa.2009.07.008
- Arjunan, V., Rani, T., Mythili, C. V., Mohan, S. (2011). Synthesis, FT-IR, FT-Raman and quantum chemical investigations of N-(3-methylphenyl)-2,2-dichloroacetamide. European Journal of Chemistry, 2 (1), 70–76. https://doi.org/10.5155/eurjchem.2.1.70-76.286
- Li, T., Yang, Y., Cheng, C., Tiwari, A. K., Sodani, K., Zhao, Y. et al. (2012). Design, synthesis and biological evaluation of N-arylphenyl-2,2-dichloroacetamide analogues as anti-cancer agents. Bioorganic & Medicinal Chemistry Letters, 22 (23), 7268–7271. https://doi.org/10.1016/j.bmcl.2012.07.057
- Li, T. W., Yang, Y. C., Cheng, C. M., Wang, D. C., Lu, A. J., Zhao, Y. F. (2012). Multi-substituted N-phenyl-2, 2-dichloroacetamide analogues as anti-cancer drugs: design, synthesis, and biological evaluation. Yao xue xue bao = Acta pharmaceutica Sinica, 47 (3), 354–363.
- Yang, Y., Shang, P., Cheng, C., Wang, D., Yang, P., Zhang, F. et al. (2010). Novel N-phenyl dichloroacetamide derivatives as anticancer reagents: Design, synthesis and biological evaluation. European Journal of Medicinal Chemistry, 45 (9), 4300–4306. https://doi.org/10.1016/j.ejmech.2010.06.032
- Porrès, L., Mongin, O., Katan, C., Charlot, M., Pons, T., Mertz, J., Blanchard-Desce, M. (2003). Enhanced Two-Photon Absorption with Novel Octupolar Propeller-Shaped Fluorophores Derived from Triphenylamine. Organic Letters, 6 (1), 47–50. https://doi.org/10.1021/ol036041s
- Fereidoonnezhad, M., Faghih, Z., Mojaddami, A., Tabaei, S. M. H., Rezaei, Z. (2016). Novel approach synthesis, molecular docking, and cytotoxic activity evaluation of N-phenyl-2,2-dichloroacetamide derivatives as anticancer agents. Journal of Sciences, Islamic Republic of Iran, 27 (1), 39–49.
- Havryshchuk, L. M., Horishny, V. Y., Lesyk, R. B. (2022). (2022). Synthesis of dichloroacetamides and study of their anti-tumor activity. Farmatsevtychnyi Zhurnal, 4, 42–49. https://doi.org/10.32352/0367-3057.4.22.05
- Zhang, S.-L., Zhang, W., Xiao, Q., Yang, Z., Hu, X., Wei, Z., Tam, K. Y. (2016). Development of dichloroacetamide pyrimidines as pyruvate dehydrogenase kinase inhibitors to reduce cancer cell growth: synthesis and biological evaluation. RSC Advances, 6 (82), 78762–78767. https://doi.org/10.1039/c6ra14060b
- Norman, M. H., Chen, N., Chen, Z., Fotsch, C., Hale, C., Han, N. et al. (2000). Structure-Activity Relationships of a Series of Pyrrolo[3,2-d]pyrimidine Derivatives and Related Compounds as Neuropeptide Y5 Receptor Antagonists. Journal of Medicinal Chemistry, 43 (22), 4288–4312. https://doi.org/10.1021/jm000269t
- Baindur, N., Chadha, N., Player, M. R. (2003). Solution-Phase Synthesis of a Library of 3,5,7-Trisubstituted 3H-[1,2,3]triazolo[4,5-d]pyrimidines. Journal of Combinatorial Chemistry, 5 (5), 653–659. https://doi.org/10.1021/cc020110x
- Uden, P. C., Miller, J. W. (1983). Chlorinated acids and chloral in drinking water. Journal AWWA, 75 (10), 524–527. https://doi.org/10.1002/j.1551-8833.1983.tb05213.x
- Mughal, F. H. (1992). Chlorination of drinking water and cancer: a review. Journal of environmental pathology, toxicology, and oncology: official organ of the International Society for Environmental Toxicology and Cancer, 11 (5-6), 287–292.
- Y Yan, Z., Henderson, G. N., James, M. O., Stacpoole, P. W. (1997). Determination of dichloroacetate and its metabolites in human plasma by gas chromatography–mass spectrometry. Journal of Chromatography B: Biomedical Sciences and Applications, 703 (1-2), 75–84. https://doi.org/10.1016/s0378-4347(97)00404-0
- Stacpoole, P. W., Henderson, G. N., Yan, Z., James, M. O. (1998). Clinical pharmacology and toxicology of dichloroacetate. Environmental Health Perspectives, 106 (4), 989–994. https://doi.org/10.1289/ehp.98106s4989
- Dichloressigsäure und ihre Salze [MAK Value Documentation in German language, 2010] (2012). The MAK-Collection for Occupational Health and Safety, 1-136. https://doi.org/10.1002/3527600418.mb7943verd0049
- Hartwig, A. (2021). Dichloroacetic acid and its salts: MAK Value Documentation, supplement – Translation of the German version from 2019. MAK Commission. Wiley-VCH Verlag.
- Cornett, R., James, M. O., Henderson, G. N., Cheung, J., Shroads, A. L., Stacpoole, P. W. (1999). Inhibition of glutathione S-transferase zeta and tyrosine metabolism by dichloroacetate: a potential unifying mechanism for its altered biotransformation and toxicity. Biochemical and biophysical research communications, 262 (3), 752–756. https://doi.org/10.1006/bbrc.1999.1287
- Shroads, A. L., Guo, X., Dixit, V., Liu, H.-P., James, M. O., Stacpoole, P. W. (2007). Age-Dependent Kinetics and Metabolism of Dichloroacetate: Possible Relevance to Toxicity. Journal of Pharmacology and Experimental Therapeutics, 324 (3), 1163–1171. https://doi.org/10.1124/jpet.107.134593
- Jordi, A. D., Alhelí, R. C., Alba, M. F., Luís, I. (2015). Pat. No. WO2015135926A1. Dichloroacetate compounds for use in treating a disease caused by a glycolytic parasite. Univ Barcelona Autonoma [ES]; published: 17.09.2015.
- Madhok, B. M., Yeluri, S., Perry, S. L., Hughes, T. A., Jayne, D. G. (2010). Dichloroacetate induces apoptosis and cell-cycle arrest in colorectal cancer cells. British Journal of Cancer, 102(12), 1746–1752. https://doi.org/10.1038/sj.bjc.6605701
- Stockwin, L. H., Yu, S. X., Borgel, S., Hancock, C., Wolfe, T. L., Phillips, L. R. et al. (2010). Sodium dichloroacetate selectively targets cells with defects in the mitochondrial ETC. International Journal of Cancer, 127 (11), 2510–2519. https://doi.org/10.1002/ijc.25499
- Soo, K. K., Jun, P. Y., Hyun-Nam, S., Youn, K. D., Woo, K. J., Kyoung-Suk, C. et al. (2012). Pat. No. WO2012008711A2. Erlotinib dichloroacetate and anti-cancer agent comprising the same. Celltrion Chemical Research Institute [Korea]; published: 19.01.2012.
- Abdelmalak, M., Lew, A., Ramezani, R., Shroads, A. L., Coats, B. S., Langaee, T. et al. (2013). Long-term safety of dichloroacetate in congenital lactic acidosis. Molecular Genetics and Metabolism, 109 (2), 139–143. https://doi.org/10.1016/j.ymgme.2013.03.019
- Stacpoole, P. W., Moore, G. W., Kornhauser, D. M. (1978). Metabolic Effects of Dichloroacetate in Patients with Diabetes Mellitus and Hyperlipoproteinemia. New England Journal of Medicine, 298 (10), 526–530. https://doi.org/10.1056/nejm197803092981002
- Feldhoff, R. C., Taylor, J. M., Jefferson, L. S. (1977). Synthesis and secretion of rat albumin in vivo, in perfused liver, and in isolated hepatocytes. Effects of hypophysectomy and growth hormone treatment. Journal of Biological Chemistry, 252 (11), 3611–3616. https://doi.org/10.1016/s0021-9258(17)40296-1
- Misbin, R. I. (1979). Effects of Dichloroacetate on Lipid Metabolism in Isolated Rat Liver Cells. Diabetes, 28 (4), 265–271. https://doi.org/10.2337/diab.28.4.265
- Bonnet, S., Archer, S. L., Allalunis-Turner, J., Haromy, A., Beaulieu, C., Thompson, R. et al. (2007). A Mitochondria-K+ Channel Axis Is Suppressed in Cancer and Its Normalization Promotes Apoptosis and Inhibits Cancer Growth. Cancer Cell, 11 (1), 37–51. https://doi.org/10.1016/j.ccr.2006.10.020
- Bowker-Kinley, M. M., Davis, I. W., Wu, P., Harris, A. R., Popov, M. K. (1998). Evidence for existence of tissue-specific regulation of the mammalian pyruvate dehydrogenase complex. Biochemical Journal, 329 (1), 191–196. https://doi.org/10.1042/bj3290191
- Knoechel, T. R., Tucker, A. D., Robinson, C. M., Phillips, C., Taylor, W., Bungay, P. J. et al. (2005). Regulatory Roles of the N-Terminal Domain Based on Crystal Structures of Human Pyruvate Dehydrogenase Kinase 2 Containing Physiological and Synthetic Ligands,. Biochemistry, 45 (2), 402–415. https://doi.org/10.1021/bi051402s
- Michelakis, E. D., Webster, L., Mackey, J. R. (2008). Dichloroacetate (DCA) as a potential metabolic-targeting therapy for cancer. British Journal of Cancer, 99 (7), 989–994. https://doi.org/10.1038/sj.bjc.6604554
- Stacpoole, P. W., Harwood, H. J., Jr, Cameron, D. F., Curry, S. H., Samuelson, D. A., Cornwell, P. E., Sauberlich, H. E. (1990). Chronic toxicity of dichloroacetate: possible relation to thiamine deficiency in rats. Fundamental and applied Toxicology: official journal of the Society of Toxicology, 14 (2), 327–337. https://doi.org/10.1016/0272-0590(90)90212-3
- Spruijt, L., Naviaux, R. K., McGowan, K. A., Nyhan, W. L., Sheean, G., Haas, R. H., Barshop, B. A. (2001). Nerve conduction changes in patients with mitochondrial diseases treated with dichloroacetate. Muscle & Nerve, 24 (7), 916–924. https://doi.org/10.1002/mus.1089
- Flavin, D. F. (2010). Non-Hodgkin’s Lymphoma Reversal with Dichloroacetate. Journal of Oncology, 2010, 1–4. https://doi.org/10.1155/2010/414726
- Khan, A. (2013). Case Report of Long Term Complete Remission of Metastatic Renal Squamous Cell Carcinoma after Palliative Radiotherapy and Adjuvant Dichloroacetate. Advances in Cancer: Research & Treatment, 1–7. https://doi.org/10.5171/2012.441895
- Corraliza, I. M., Campo, M. L., Soler, G., Modolell, M. (1994). Determination of arginase activity in macrophages: a micromethod. Journal of Immunological Methods, 174 (1-2), 231–235. https://doi.org/10.1016/0022-1759(94)90027-2
- Azuma, M., Ebihara, T., Oshiumi, H., Matsumoto, M., Seya, T. (2012). Cross-priming for antitumor CTL induced by soluble Ag + polyI:C depends on the TICAM-1 pathway in mouse CD11c+/CD8α+dendritic cells. OncoImmunology, 1 (5), 581–592. https://doi.org/10.4161/onci.19893
- Akazawa, T., Ebihara, T., Okuno, M., Okuda, Y., Shingai, M., Tsujimura, K. et al. (2007). Antitumor NK activation induced by the Toll-like receptor 3-TICAM-1 (TRIF) pathway in myeloid dendritic cells. Proceedings of the National Academy of Sciences, 104 (1), 252–257. https://doi.org/10.1073/pnas.0605978104
- Ohashi, T., Akazawa, T., Aoki, M., Kuze, B., Mizuta, K., Ito, Y., Inoue, N. (2013). Dichloroacetate improves immune dysfunction caused by tumor‐secreted lactic acid and increases antitumor immunoreactivity. International Journal of Cancer, 133 (5), 1107–1118. https://doi.org/10.1002/ijc.28114
- Cheong, H., Lu, C., Lindsten, T., Thompson, C. B. (2012). Therapeutic targets in cancer cell metabolism and autophagy. Nature Biotechnology, 30 (7), 671–678. https://doi.org/10.1038/nbt.2285
- Abemayor, E., Kovachich, G. B., Haugaard, N. (1984). Effects of Dichloroacetate on Brain Pyruvate Dehydrogenase. Journal of Neurochemistry, 42 (1), 38–42. https://doi.org/10.1111/j.1471-4159.1984.tb09694.x
- Wong, J. Y. Y., Huggins, G. S., Debidda, M., Munshi, N. C., De Vivo, I. (2008). Dichloroacetate induces apoptosis in endometrial cancer cells. Gynecologic Oncology, 109 (3), 394–402. https://doi.org/10.1016/j.ygyno.2008.01.038
- Duan, Y., Zhao, X., Ren, W., Wang, X., Yu, K. F., Li, D. et al. (2013). Antitumor activity of dichloroacetate on C6 glioma cell: in vitro and in vivo evaluation. OncoTargets and Therapy, 6, 189–198. https://doi.org/10.2147/ott.s40992
- Kolesnik, D. L., Pyaskovskaya, O. N., Yurchenko, O. V., Solyanik, G. I. (2023). Metformin enhances antitumor action of sodium dichloroacetate against glioma C6. Experimental Oncology, 41 (2), 123–129. https://doi.org/10.32471/exp-oncology.2312-8852.vol-41-no-2.1306
- Kolesnik, D. L., Pyaskovskaya, O. N., Boichuk, I. V., Solyanik, G. I. (2014). Hypoxia enhances antitumor activity of dichloroacetate. Experimental oncology, 36 (4), 231–235.
- Seliger, C., Renner, K. (2017). P08.52 Metformin as adjuvant therapy for glioma. Neuro-Oncology, 19 (3), iii65. https://doi.org/10.1093/neuonc/nox036.241
- Quaile, M. P., Melich, D. H., Jordan, H. L., Nold, J. B., Chism, J. P., Polli, J. W. et al. (2010). Toxicity and toxicokinetics of metformin in rats. Toxicology and Applied Pharmacology, 243 (3), 340–347. https://doi.org/10.1016/j.taap.2009.11.026
- Prokhorova, I. V., Pyaskovskaya, O. N., Kolesnik, D. L., Solyanik, G. I. (2018). Influence of metformin, sodium dichloroacetate and their combination on the hematological and biochemical blood parameters of rats with gliomas C6. Experimental oncology, 40 (3), 205–210. https://doi.org/10.31768/2312-8852.2018.40(3):205-210
- Pustylnikov, S., Costabile, F., Beghi, S., Facciabene, A. (2018). Targeting mitochondria in cancer: current concepts and immunotherapy approaches. Translational Research, 202, 35–51. https://doi.org/10.1016/j.trsl.2018.07.013
- Grazioli, S., Pugin, J. (2018). Mitochondrial Damage-Associated Molecular Patterns: From Inflammatory Signaling to Human Diseases. Frontiers in Immunology, 9. https://doi.org/10.3389/fimmu.2018.00832
- Torres-Cavazos, Z., Franco-Molina, M. A., Santana-Krímskaya, S. E., Rodríguez-Padilla, C., Kawas-Garza, J. R., Hernández-Vidal, G. et al. (2020). In Vivo Evaluation of the Antitumor and Immunogenic Properties of Silver and Sodium Dichloroacetate Combination against Melanoma. Journal of Nanomaterials, 2020, 1–8. https://doi.org/10.1155/2020/3741019
- Bonner, M. Y., Karlsson, I., Rodolfo, M., Arnold, R. S., Vergani, E., Arbiser, J. L. (2016). Honokiol bis-dichloroacetate (Honokiol DCA) demonstrates activity in vemurafenib-resistant melanomain vivo. Oncotarget, 7 (11), 12857–12868. https://doi.org/10.18632/oncotarget.7289
- Vergani, E., Vallacchi, V., Frigerio, S., Deho, P., Mondellini, P., Perego, P. et al. (2011). Identification of MET and SRC Activation in Melanoma Cell Lines Showing Primary Resistance to PLX4032. Neoplasia, 13 (12), 1132-IN17. https://doi.org/10.1593/neo.111102
- Stacpoole, P. W. (1989). The pharmacology of dichloroacetate. Metabolism, 38 (11), 1124–1144. https://doi.org/10.1016/0026-0495(89)90051-6
- Stacpoole, P. W., Gilbert, L. R., Neiberger, R. E., Carney, P. R., Valenstein, E., Theriaque, D. W., Shuster, J. J. (2008). Evaluation of Long-term Treatment of Children With Congenital Lactic Acidosis With Dichloroacetate. Pediatrics, 121 (5), e1223–e1228. https://doi.org/10.1542/peds.2007-2062
- Ishiguro, T., Ishiguro, M., Ishiguro, R., Iwai, S. (2012). Cotreatment with dichloroacetate and omeprazole exhibits a synergistic antiproliferative effect on malignant tumors. Oncology Letters, 3 (3), 726–728. https://doi.org/10.3892/ol.2012.552
- Michelakis, E. D., Sutendra, G., Dromparis, P., Webster, L., Haromy, A., Niven, E. et al. (2010). Metabolic Modulation of Glioblastoma with Dichloroacetate. Science Translational Medicine, 2 (31), 31–34. https://doi.org/10.1126/scitranslmed.3000677
- Tong, J., Xie, G., He, J., Li, J., Pan, F., Liang, H. (2011). Synergistic Antitumor Effect of Dichloroacetate in Combination with 5-Fluorouracil in Colorectal Cancer. Journal of Biomedicine and Biotechnology, 2011, 1–7. https://doi.org/10.1155/2011/740564
- Longley, D. B., Harkin, D. P., Johnston, P. G. (2003). 5-Fluorouracil: mechanisms of action and clinical strategies. Nature Reviews Cancer, 3 (5), 330–338. https://doi.org/10.1038/nrc1074
- Lu, X., Zhou, D., Hou, B., Liu, Q.-X., Chen, Q., Deng, X.-F., Yu, Z.-B., Dai, J.-G., Zheng, H. (2018). Dichloroacetate enhances the antitumor efficacy of chemotherapeutic agents via inhibiting autophagy in non-small-cell lung cancer. Cancer Management and Research, 10, 1231–1241. https://doi.org/10.2147/cmar.s156530
- Lin, G., Hill, D. K., Andrejeva, G., Boult, J. K. R., Troy, H., Fong, A.-C. L. F. W. T. et al. (2014). Dichloroacetate induces autophagy in colorectal cancer cells and tumours. British Journal of Cancer, 11 1(2), 375–385. https://doi.org/10.1038/bjc.2014.281
- Gong, F., Peng, X., Sang, Y., Qiu, M., Luo, C., He, Z. et al. (2013). Dichloroacetate induces protective autophagy in LoVo cells: involvement of cathepsin D/thioredoxin-like protein 1 and Akt-mTOR-mediated signaling. Cell Death & Disease, 4 (11), e913–e913. https://doi.org/10.1038/cddis.2013.438
- Usman, M., Arjmand, F., Khan, R. A., Alsalme, A., Ahmad, M., Tabassum, S. (2017). Biological evaluation of dinuclear copper complex/dichloroacetic acid cocrystal against human breast cancer: design, synthesis, characterization, DFT studies and cytotoxicity assays. RSC Adv., 7 (76), 47920–47932. https://doi.org/10.1039/c7ra08262b
- Suh, Y., Amelio, I., Guerrero Urbano, T., Tavassoli, M. (2014). Clinical update on cancer: molecular oncology of head and neck cancer. Cell Death & Disease, 5 (1), e1018–e1018. https://doi.org/10.1038/cddis.2013.548
- Chu, Q. S.-C., Sangha, R., Spratlin, J., J. Vos, L., Mackey, J. R., McEwan, A. J. B. et al. (2015). A phase I open-labeled, single-arm, dose-escalation, study of dichloroacetate (DCA) in patients with advanced solid tumors. Investigational New Drugs, 33 (3), 603–610. https://doi.org/10.1007/s10637-015-0221-y
- Golding, J. P., Wardhaugh, T., Patrick, L., Turner, M., Phillips, J. B., Bruce, J. I., Kimani, S. G. (2013). Targeting tumour energy metabolism potentiates the cytotoxicity of 5-aminolevulinic acid photodynamic therapy. British Journal of Cancer, 109 (4), 976–982. https://doi.org/10.1038/bjc.2013.391
- Alkarakooly, Z., Al-Anbaky, Q. A., Kannan, K., Ali, N. (2018). Metabolic reprogramming by Dichloroacetic acid potentiates photodynamic therapy of human breast adenocarcinoma MCF-7 cells. PLOS ONE, 13 (10), e0206182. https://doi.org/10.1371/journal.pone.0206182
- Jackson, S. (2008). Pat. No. US2008221211A1. Method of treatment of neurological injury or cancer by administration of dichloroacetate. Published: 11.09.2008.
- Addala, E., Rafiei, H., Das, S., Bandy, B., Das, U., Karki, S. S., Dimmock, J. R. (2017). 3,5-Bis(3-dimethylaminomethyl-4-hydroxybenzylidene)-4-piperidone and related compounds induce glutathione oxidation and mitochondria-mediated cell death in HCT-116 colon cancer cells. Bioorganic & Medicinal Chemistry Letters, 27 (16), 3669–3673. https://doi.org/10.1016/j.bmcl.2017.07.018
- Warburg, O. (1956). On the Origin of Cancer Cells. Science, 123 (3191), 309–314. https://doi.org/10.1126/science.123.3191.309
- Fedorchuk, A. G., Pyaskovskaya, O. N., Gorbik, G. V., Prokhorova, I. V., Kolesnik, D. L., Solyanik, G. I. (2016). Effectiveness of sodium dichloroacetate against glioma C6 depends on administration schedule and dosage. Experimental oncology, 38 (2), 80–83. https://doi.org/10.31768/2312-8852.2016.38(2):80-83
- Tataranni, T., Piccoli, C. (2019). Dichloroacetate (DCA) and Cancer: An Overview towards Clinical Applications. Oxidative Medicine and Cellular Longevity, 2019, 1–14. https://doi.org/10.1155/2019/8201079
- Hossain, M., Das, S., Das, U., Doroudi, A., Zhu, J., Dimmock, J. R. (2020). Novel hybrid molecules of 3,5-bis(benzylidene)-4-piperidones and dichloroacetic acid which demonstrate potent tumour-selective cytotoxicity. Bioorganic & Medicinal Chemistry Letters, 30 (3), 126878. https://doi.org/10.1016/j.bmcl.2019.126878
- James, M. O., Yan, Z., Cornett, R., Jayanti, V. M., Henderson, G. N., Davydova, N. et al. (1998). Pharmacokinetics and metabolism of [14C]dichloroacetate in male Sprague-Dawley rats. Identification of glycine conjugates, including hippurate, as urinary metabolites of dichloroacetate. Drug metabolism and disposition: the biological fate of chemicals, 26 (11), 1134–1143.
- James, M. O., Jahn, S. C., Zhong, G., Smeltz, M. G., Hu, Z., Stacpoole, P. W. (2017). Therapeutic applications of dichloroacetate and the role of glutathione transferase zeta-1. Pharmacology & Therapeutics, 170, 166–180. https://doi.org/10.1016/j.pharmthera.2016.10.018
- Dhar, S., Lippard, S. J. (2009). Mitaplatin, a potent fusion of cisplatin and the orphan drug dichloroacetate. Proceedings of the National Academy of Sciences, 106 (52), 22199–22204. https://doi.org/10.1073/pnas.0912276106
- Stacpoole, P. W., Harwood, H. J., Varnado, C. E. (1983). Regulation of rat liver hydroxymethylglutaryl coenzyme A reductase by a new class of noncompetitive inhibitors. Effects of dichloroacetate and related carboxylic acids on enzyme activity. Journal of Clinical Investigation, 72 (5), 1575–1585. https://doi.org/10.1172/jci111116
- Stacpoole, P. W., Gonzalez, M. G., Vlasak, J., Oshiro, Y., Bodor, N. (1987). Dichloroacetate derivatives. metabolic effects and pharmacodynamics in normal rats. Life Sciences, 41 (18), 2167–2176. https://doi.org/10.1016/0024-3205(87)90535-2
- Kasenov, K. Zh. (2012). Transdermalnye terapevticheskie sistemy – chreskozhnaia dostavka lekarstvennykh veshchestv (obzor). Klinicheskaia meditcina Kazakhstana, 1 (24), 110–115.
- Dhar, S., Pathak, R. (2018). Pat. No. US10004809B2. Precise delivery of therapeutic agents to cell mitochondria for anti-cancer therapy. University of Georgia Research Foundation Inc UGARF (USA); published: 26.06.2018.
- Soo, K. K., Jun, P. Y., Hyun-Nam, S., Woo, K. J. (2012). Pat. No. US2012295917A1. Imatinib dichloroacetate and anti-cancer agent comprising the same. Celltrion Chemical Res INST [KR]; published: 22.11.2012.
- Li, B. (2011). Pat. No. CN101984967A. Manganoporphyrin-dichloroacetic acid combined medicament for treating tumors. Shandong Hongli Lab Animal Experiment Co LTD (СN); published: 16.03.2011.
- Evangelos, M., Stephen, A. (2006). Pat. No. WO2006108276A1. A method of treating cancer using dichloroacetate. Published: 19.10.2006.
- Jianping, D., Quan, L. (2017). Pat. No. CN106986791A. Medicinal compound for treating tumors and preparation method and application thereof. Published: 28.07.2017.
- Soo-Youl, K., Jong-Heon, K., Young-Ki, B., Ho, L., Hyon-Chol, J., Beom-Kyu, C. et al. (2016). Pat. No. CN106232109A. Pharmaceutical composition for cancer treatment containing gossypol and phenformin as active ingredients. Published 14.12.2016.
- Boyd, M. R., Paull, K. D. (1995). Some practical considerations and applications of the national cancer institute in vitro anticancer drug discovery screen. Drug Development Research, 34 (2), 91–109. https://doi.org/10.1002/ddr.430340203
- Alley, M. C., Scudiero, D. A., Monks, A., Hursey, M. L., Czerwinski, M. J., Fine, D. L. et al. (1988). Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Research, 48 (3), 589–601.
- Shoemaker, R. H. (2006). The NCI60 human tumour cell line anticancer drug screen. Nature Reviews Cancer, 6 (10), 813–823. https://doi.org/10.1038/nrc1951
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Liubomyr Havryshchuk, Volodymyr Horishny, Nadiіa Rushchak, Roman Lesyk

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






