Assessment of the anti-psoriasis effect of Scrophularia deserti methanolic extract in mice model
DOI:
https://doi.org/10.15587/2519-4852.2024.299266Keywords:
anti-psoriasis, Scrophularia deserti, methanolic, extract, antioxidant, phenols, flavonoids, cytokine, Imiquimod, mice modelAbstract
Psoriasis is an underestimated chronic and autoimmune skin disorder. Topical chemical agents are applied for psoriasis control and treatment, notwithstanding their subordinate efficiency or unsuccessful activities. As an alternative, herbal medicine can also be used in its treatment.
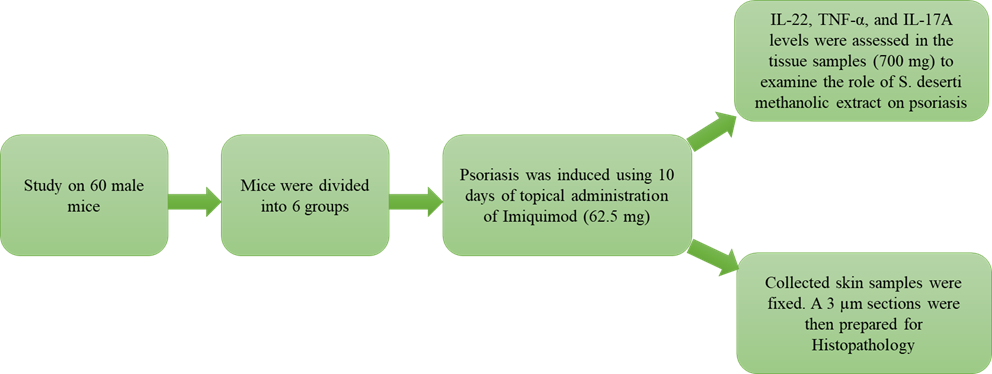
The aim of the present study was performed to assess the anti-psoriasis effect of Scrophularia deserti in mice model.
Materials and methods: S. deserti was purchased and used for methanolic extraction. Extract DPPH radical scavenging activity, polyphenol and flavonoid contents were examined. Sixty male mice were purchased, and psoriasis was induced using 10 days of topical administration of Imiquimod (62.5 mg). Mice were classified into 6 groups: non-psoriasis control (only received distilled water), psoriasis control (only received topical Imiquimod), two S. deserti treatments (topical 300 and 500 mg/kg), topical Betamethasone, and topical α-pinene 9 %. Cytokine distribution and histopathological properties were also determined.
Results: the value at which the S. deserti methanolic extract scavenges 50 % of free radicals (IC50) was 602.71±15.33 µg/mL. The total S. deserti methanolic extract flavonoid and polyphenol contents were 16.85±1.12 mg QE/g and 58.47±3.25 mg GAE/g, respectively. IL-22, TNF-α, and IL-17A concentrations increased after psoriasis induction compared to the control group (P <0.05). Mice treated with Betamethasone harboured the lowest concentrations of IL-22, TNF-α, and IL-17A (P <0.05).
Conclusions: Mice treated with S. deserti methanolic extract (500 mg/kg) also harboured significantly lower IL-22, TNF-α, and IL-17A (P <0.05) compared to α-pinene and S. deserti methanolic extract (300 mg/kg). Mice of the psoriasis control group showed significant epidermis hyperkeratosis, acanthosis, and crust with plentiful inflammatory cells. At the same time, mice treated with S. deserti methanolic extract (500 mg/kg) showed significant recovered tissue with normal skin epidermis and dermis, sebaceous glands, and follicles of the hair, besides the lowest rate of inflammatory reactions. Findings showed that the S. deserti methanolic extract (500 mg/kg) can efficiently be used as a practical substitute for psoriasis treatment. However, some supplementary research should be performed
References
- Schön, M. P., Wilsmann‐Theis, D. (2023). Current developments and perspectives in psoriasis. JDDG: Journal Der Deutschen Dermatologischen Gesellschaft, 21 (4), 363–372. https://doi.org/10.1111/ddg.15033
- Potestio, L., Ruggiero, A., Fabbrocini, G., Martora, F., Megna, M. (2023). Effectiveness and Safety of Deucravacitinib for the Management of Psoriasis: A Review of the Current Literature. Psoriasis: Targets and Therapy, Volume 13, 19–26. https://doi.org/10.2147/ptt.s407647
- Man, A.-M., Orăsan, M. S., Hoteiuc, O.-A., Olănescu-Vaida-Voevod, M.-C., Mocan, T. (2023). Inflammation and Psoriasis: A Comprehensive Review. International Journal of Molecular Sciences, 24 (22), 16095. https://doi.org/10.3390/ijms242216095
- Nicolescu, A. C., Ionescu, M.-A., Constantin, M. M., Ancuta, I., Ionescu, S., Niculet, E. et al. (2022). Psoriasis Management Challenges Regarding Difficult-to-Treat Areas: Therapeutic Decision and Effectiveness. Life, 12 (12), 2050. https://doi.org/10.3390/life12122050
- Greb, J. E., Goldminz, A. M., Elder, J. T., Lebwohl, M. G., Gladman, D. D., Wu, J. J. et al. (2016). Psoriasis. Nature Reviews Disease Primers, 2 (1). https://doi.org/10.1038/nrdp.2016.82
- Lee, H.-J., Kim, M. (2023). Challenges and Future Trends in the Treatment of Psoriasis. International Journal of Molecular Sciences, 24 (17), 13313. https://doi.org/10.3390/ijms241713313
- Khaleel, R. A., Shareef, S. M., Hameed, Z. E., Alsaraf, K. M., Nassar, M. F. (2021). The effect of fluoxetine and imipramine on the improvement of depressive-like behaviors and HPA axis (hypothalamic-pituitary-adrenal cortex) activity – an animal model. ScienceRise: Pharmaceutical Science, 5 (33), 79–88. https://doi.org/10.15587/2519-4852.2021.243526
- Mardani, M., Rezapour, S., Eftekhari, Z., Asadi-Samani, M., Rashidipour, M., Afsordeh, O. et al. Chemical composition of Elamit scrophularia deserti. International Journal of PharmTech Research, 9 (6), 285–290.
- Zarshenas, M. M., Mousavi, S. S., Haghighi, T. M. (2022). A critical overview of Scrophularia striata Boiss.: Phytochemical and pharmacological investigations. Pharmacological Research – Modern Chinese Medicine, 5, 100182. https://doi.org/10.1016/j.prmcm.2022.100182
- Mahmoud, B., Hadavi, M., Abbasi, N. (2020). Study of extraction and chemical compounds of Scrophularia striata Boiss. and Scrophularia deserti Delile using HS-SPME and GC-MS. Plant Biotechnology Persa, 2 (1), 8–13. https://doi.org/10.29252/pbp.2.1.8
- Singleton, V. L., Orthofer, R., Lamuela-Raventós, R. M. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods in Enzymology, 299, 152–178. https://doi.org/10.1016/s0076-6879(99)99017-1
- Shareef, S. M., Khaleel, R. A., Hameed, Z. E., Alsaraf, K. M. (2021). The protective effect of Zingiber officinale L. extract on kidney tissues and blood factors of kidney functions after the damage caused by Azathioprine. ScienceRise: Pharmaceutical Science, 4(32), 78–86. https://doi.org/10.15587/2519-4852.2021.239434
- Pang, X., Zhang, K., Huang, J., Wang, H., Gao, L., Wang, T. et al. (2018). Decryption of Active Constituents and Action Mechanism of the Traditional Uighur Prescription (BXXTR) Alleviating IMQ-Induced Psoriasis-Like Skin Inflammation in BALB/c Mice. International Journal of Molecular Sciences, 19 (7), 1822. https://doi.org/10.3390/ijms19071822
- Pirowska, M., Podolec, K., Lipko-Godlewska, S., Sułowicz, J., Brzewski, P., Obtułowicz, A. et al. (2019). Level of inflammatory cytokines tumour necrosis factor, interleukins 12, 23 and 17 in patients with psoriasis in the context of metabolic syndrome. Advances in Dermatology and Allergology, 36 (1), 70–75. https://doi.org/10.5114/ada.2018.73136
- Elkhawaga, O. Y., Ellety, M. M., Mofty, S. O., Ghanem, M. S., & Mohamed, A. O. (2023). Review of natural compounds for potential psoriasis treatment. Inflammopharmacology, 31 (3), 1183–1198. https://doi.org/10.1007/s10787-023-01178-0
- Bezuglaya, E., Stolper, Y., Lyapunov, N., Zinchenko, I., Liapunov, O. (2023). Study of factors affecting some properties of hydrophilic suppository base. ScienceRise: Pharmaceutical Science, 5 (45), 4–15. https://doi.org/10.15587/2519-4852.2023.286315
- Aghmiuni, A. I., Khiavi, A. A. (2017). Medicinal Plants to Calm and Treat Psoriasis Disease. Aromatic and Medicinal Plants – Back to Nature, 1–28. https://doi.org/10.5772/67062
- Nowak-Perlak, M., Szpadel, K., Jabłońska, I., Pizon, M., Woźniak, M. (2022). Promising Strategies in Plant-Derived Treatments of Psoriasis-Update of In Vitro, In Vivo, and Clinical Trials Studies. Molecules, 27 (3), 591. https://doi.org/10.3390/molecules27030591
- Pasdaran, A., Hamedi, A. (2017). The genus Scrophularia: a source of iridoids and terpenoids with a diverse biological activity. Pharmaceutical Biology, 55 (1), 2211–2233. https://doi.org/10.1080/13880209.2017.1397178
- Borodina, N., Maloshtan, L., Artemova, K., Kukhtenko, O. (2023). Study of pharmacological activity of dry extract of sakhalin willow shoots against the background of experimental thrombophlebitis. ScienceRise: Pharmaceutical Science, 4 (44), 97–103. https://doi.org/10.15587/2519-4852.2023.286723
- Tanideh, N, Haddadi, M. H., Rokni Hosseini, M. H., Hossienzadeh, M., Mehrabani, D., Sayehmiri, K., Koohi-Hossienabadi, O. (2015). The healing effect of Scrophularia striata on experimental burn wounds infected to Pseudomonas aeruginosa in rat. World Journal of Plastic Surgery, 4, 16–23.
- Jafary, A., Latifi, A., Shohrati, M., Haji Hosseini, R., Salesi, M. (2013). The effect of Scrophularia striata extracts on wound healing of mice. Armaghane Danesh, 18, 194–209
- Haddadi, R., Tamri, P., Javani Jooni, F. (2019). In vitro wound healing activity of Scrophularia striata hydroalcoholic extract. South African Journal of Botany, 121, 505–509. https://doi.org/10.1016/j.sajb.2019.01.002
- Dı́az, A. M., Abad, M. J., Fernández, L., Silván, A. M., De Santos, J., Bermejo, P. (2004). Phenylpropanoid glycosides from Scrophularia scorodonia: In vitro anti-inflammatory activity. Life Sciences, 74 (20), 2515–2526. https://doi.org/10.1016/j.lfs.2003.10.008
- Ghashghaii, A., Hashemnia, M., Nikousefat, Z., Zangeneh, M. M., Zangeneh, A. (2017). Wound Healing Potential of Methanolic Extract of Scrophularia striata in Rats. Pharmaceutical Sciences, 23 (4), 256–263. https://doi.org/10.15171/ps.2017.38
- Sabahi, M. R., Taghipour, M., Nouredini, M., Javadi, S. M. (2020). Evaluation of wound healing effects of Scrophularia striata seed extract in rat. International Journal of Medical Investigation, 9 (1), 20–28.
- Zengin, G., Stefanucci, A., Rodrigues, M. J., Mollica, A., Custodio, L., Aumeeruddy, M. Z., Mahomoodally, M. F. (2019). Scrophularia lucida L. as a valuable source of bioactive compounds for pharmaceutical applications: In vitro antioxidant, anti-inflammatory, enzyme inhibitory properties, in silico studies, and HPLC profiles. Journal of Pharmaceutical and Biomedical Analysis, 162, 225–233. https://doi.org/10.1016/j.jpba.2018.09.035
- Xu, Y., Shi, Y., Huang, J., Gu, H., Li, C., Zhang, L., Liu, G., Zhou, W., Du, Z. (2022). The Essential Oil Derived from Perilla frutescens (L.) Britt. Attenuates Imiquimod-Induced Psoriasis-like Skin Lesions in BALB/c Mice. Molecules, 27 (9), 2996. https://doi.org/10.3390/molecules27092996
- Lee, J. H., Lee, M.-Y. (2023). In Vitro and In Vivo Anti-Psoriasis Activity of Ficus carica Fruit Extracts via JAK-STAT Modulation. Life, 13 (8), 1671. https://doi.org/10.3390/life13081671
- Khaledi, M., Sharif Makhmal Zadeh, B., Rezaie, A., Nazemi, M., Safdarian, M., Nabavi, M. B. (2020). Chemical profiling and anti-psoriatic activity of marine sponge (Dysidea avara) in induced imiquimod-psoriasis-skin model. PLOS ONE, 15 (11), e0241582. https://doi.org/10.1371/journal.pone.0241582
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Raghad Abdulsalam Khaleel, Saja Majeed Shareef, Tayf Mohammed Maryoosh

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






