Research for the selection of taste corrigents, filter materials and primary packaging for oral solution with magnesium salts
DOI:
https://doi.org/10.15587/2519-4852.2024.302066Keywords:
oral solution, organic magnesium salts, excipients, single-dose primary packagingAbstract
The aim. Theoretically and experimentally, justify the choice of excipients for a combined oral solution with organic magnesium salts. Determine the compatibility of filter materials of three types. Select single-dose primary packaging for the developed oral solution and confirm its suitability during the relevant studies.
Materials and methods. Organoleptic, physicochemical, and pharmaco-technological methods were used in the investigation. All methods meet the requirements of the State Pharmacopoeia of Ukraine and the European Pharmacopoeia. Organoleptic methods indicated the taste of the medicinal speciality behind the methods of O.I. Tentsova and I.A. Egorov. Physicochemical methods were used to determine pH, colour, density, and quantitative amount of active pharmaceutical ingredients. Pharmaco-technological methods were used to determine the properties of filter materials and primary packaging.
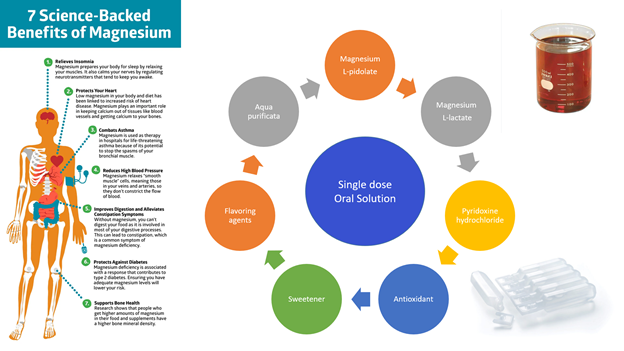
Results. A sweetener and a flavouring agent for an oral solution with magnesium salts were selected based on the research. Saccharin sodium was selected as a sweetener in the amount of 0.15 %. «Cherry» in the amount of 0.4 % and «caramel» in the amount of 0.2 % were selected as flavouring agents. As a result of experiments, the suitability of filter materials made of сapron, nylon and polyethersulfone was proven. This is determined by the constancy of the main quality indicators of the drug 24, 48 and 72 hours after filtration. The suitability of the single-dose primary packaging in the process of storing the oral solution has been studied and proven. In the work, polymer ampoules of the «Moplen EP 2S 12 B» brand and the «Purell HP 371P» brand were used. The conducted research allows us to create a competitive domestic drug that will be technologically simple and convenient in administration and will also be distinguished by relatively low raw material costs and production.
Conclusions. Based on theoretical and experimental studies, auxiliary substances, such as sweeteners and flavouring agents, were selected for the combined magnesium-containing drug. The suitability of filter materials for the combined oral solution was investigated and confirmed. The suitability of single-dose primary packaging of two types was selected and experimentally proven
References
- Wolf, M. T. F. (2017). Inherited and acquired disorders of magnesium homeostasis. Current Opinion in Pediatrics, 29 (2), 187–198. https://doi.org/10.1097/mop.0000000000000450
- Ahmed, F., Mohammed, A. (2019). Magnesium: The Forgotten Electrolyte – A Review on Hypomagnesemia. Medical Sciences, 7 (4), 56. https://doi.org/10.3390/medsci7040056
- Ismail, A. A. A., Ismail, Y., Ismail, A. A. (2017). Chronic magnesium deficiency and human disease; time for reappraisal? QJM: An International Journal of Medicine, 111 (11), 759–763. https://doi.org/10.1093/qjmed/hcx186
- Rylander, R. (2014). Bioavailability of magnesium salts. A review. Journal of Pharmacy and Nutrition Sciences, 4, 57–59. https://doi.org/10.6000/1927-5951.2014.04.01.8
- Firoz, M., Graber, M. (2002). Bioavailability of us commercial magnesium preparations. Magnesium Research, 14, 257–262.
- Blancquaert, L., Vervaet, C., Derave, W. (2019). Predicting and Testing Bioavailability of Magnesium Supplements. Nutrients, 11 (7), 1663. https://doi.org/10.3390/nu11071663
- Coudray, С., Rambeau, M., Feillet-Coudray, C., Gueux, E., Tressol, J., Mazur, A. et al. (2006). Study of magnesium bioavailability from ten organic and inorganic Mg salts in Mg-depleted rats using a stable isotope approach. Magnesium research, 18 (4), 215–223.
- Decollogne, S., Tomas, A., Lecerf, C., Adamowicz, E., Seman, M. (1997). NMDA receptor complex blockade by oral administration of magnesium: Comparison with MK-801. Pharmacology Biochemistry and Behavior, 58, 261–268. https://doi.org/10.1016/s0091-3057(96)00555-2
- Kyselovič, J., Chomanicová, N., Adamičková, A., Valášková, S., Šalingová, B., Gažová, A. (2021). A new Caco-2 cell model of in vitro intestinal barrier: application for the evaluation of magnesium salts absorption. Physiological Research, 70 (1), 31–41. https://doi.org/10.33549/physiolres.934772
- Romeo, M., Cazzaniga, A., Maier, J. (2019). Magnesium and the blood-brain barrier in vitro: effects on permeability and magnesium transport. Magnesium Research, 32 (1), 16–24.
- Kompendium. Available at: https://compendium.com.ua/uk/
- Snehyrova, D. V., Almakaieva, L. H. (2019). Vybir tekhnolohichnykh parametriv vyhotovlennia oralnoho rozchynu na osnovi solei mahniiu laktatu i mahniiu pidolatu. Farmakom, 1/2, 48–54.
- Alqahtani, M. S., Kazi, M., Alsenaidy, M. A., Ahmad, M. Z. (2021). Advances in Oral Drug Delivery. Frontiers in Pharmacology, 12. https://doi.org/10.3389/fphar.2021.618411
- Schuchardt, J. P., Hahn, A. (2017). Intestinal Absorption and Factors Influencing Bioavailability of Magnesium- An Update. Current Nutrition & Food Science, 13 (4), 260–278. https://doi.org/10.2174/1573401313666170427162740
- Noah, L., Dye, L., Bois De Fer, B., Mazur, A., Pickering, G., Pouteau, E. (2021). Effect of magnesium and vitamin B6 supplementation on mental health and quality of life in stressed healthy adults: Post‐hoc analysis of a randomised controlled trial. Stress and Health, 37 (5), 1000–1009. https://doi.org/10.1002/smi.3051
- Lawless, H. T., Rapacki, F., Horne, J., Hayes, A. (2003). The taste of calcium and magnesium salts and anionic modifications. Food Quality and Preference, 14 (4), 319–325. https://doi.org/10.1016/s0950-3293(02)00128-3
- Kim, M. E., Oleinikova, T. A., Evseev, S. B. (2015). Siropy s fitopreparatami: nomenklatura, razrabotka, osobennosti sostava, tekhnologii (obzor). Aktualnye problemy gummanitarnykh i obshchestvennykh nauk, 2, 193–198.
- Derzhavne pidpryiemstvo «Ukrainskyi naukovyi farmakopeinyi tsentr yakosti likarskykh zasobiv» (2014–2015). Derzhavna Farmakopeia Ukrainy. Kharkiv: Derzhavne pidpryiemstvo «Ukrainskyi naukovyi farmakopeinyi tsentr yakosti likarskykh zasobiv».
- European Directorate for the Quality of Medicines (EDQM) (2016). European Pharmacopoeia. Council of Europe, 67075 Strasbourg Cedex, France, 4016 p.
- Derzhavna Farmakopeia Ukrainy. Vol. 2 (2014). Kharkiv: Derzhavne pidpryiemstvo «Ukrainskyi naukovyi farmakopeinyi tsentr yakosti likarskykh zasobiv», 724.
- Anurova, M. N., Bakhrushina, E. O., Pyatigorskaia, N. V., Yambikova, O. M. (2015). the Principles of taste masking of oral gels with synthetic drugs. Pharmacy & Pharmacology, 3 (4 (11)), 15–20. https://doi.org/10.19163/2307-9266-2015-3-4(11)-15-20
- Domitrz, I., Cegielska, J. (2022). Magnesium as an Important Factor in the Pathogenesis and Treatment of Migraine – From Theory to Practice. Nutrients, 14 (5), 1089. https://doi.org/10.3390/nu14051089
- Ranade, V. V., Somberg, J. C. (2001). Bioavailability and Pharmacokinetics of Magnesium After Administration of Magnesium Salts to Humans. American Journal of Therapeutics, 8 (5), 345–357. https://doi.org/10.1097/00045391-200109000-00008
- Maier, J. A., Pickering, G., Giacomoni, E., Cazzaniga, A., Pellegrino, P. (2020). Headaches and Magnesium: Mechanisms, Bioavailability, Therapeutic Efficacy and Potential Advantage of Magnesium Pidolate. Nutrients, 12 (9), 2660. https://doi.org/10.3390/nu12092660
- Snegirev, V. P., Iakovleva, L. V., Snegireva, D. V., Almakaeva, L. G. (2018). Soedineniia magniia: lekarstvennye sredstva, ikh potreblenie i perspektivy sozdaniia novogo preparata. Chast 1. 100 magniisoderzhashchikh lekarstvennykh preparatov ukrainskogo farmatcevticheskogo rynka. Vestnik farmatcii, 78 (4), 33–43.
- Friedman, M., Levin, C. E. (2011). Nutritional and medicinal aspects of d-amino acids. Amino Acids, 42 (5), 1553–1582. https://doi.org/10.1007/s00726-011-0915-1
- Sariev, A., Lunshina, E., Zherdev, V., Mirzoian, N. (2006). Vzaimosviaz farmakokinetiki i farmakodinamiki kombinirovannogo preparata, soderzhashchego pirrolidon i piroglutaminovuiu kislotu. Eksperimentalnaia klinicheskaia fyrmakologiia, 69 (2), 58–61.
- Mennella, J. A., Spector, A. C., Reed, D. R., Coldwell, S. E. (2013). The Bad Taste of Medicines: Overview of Basic Research on Bitter Taste. Clinical Therapeutics, 35 (8), 1225–1246. https://doi.org/10.1016/j.clinthera.2013.06.007
- Wei, Y., Nedley, M. P., Bhaduri, S. B., Bredzinski, X., Boddu, S. H. S. (2014). Masking the Bitter Taste of Injectable Lidocaine HCl Formulation for Dental Procedures. AAPS PharmSciTech, 16 (2), 455–465. https://doi.org/10.1208/s12249-014-0239-z
- Cherian, S., Lee, B. S., Tucker, R. M., Lee, K., Smutzer, G. (2018). Toward Improving Medication Adherence: The Suppression of Bitter Taste in Edible Taste Films. Advances in Pharmacological Sciences, 2018, 1–11. https://doi.org/10.1155/2018/8043837
- Mesut, B., Özsoy, Y., Aksu, B. (2015). The place of drug product critical quality parameters in quality by design (QBD). Turkish Journal of Pharmaceutical Sciences, 12 (1), 75–92.
- Pillai, S., Chobisa, D., Urimi, D., Ravindra, N. (2016). Filters and filtration: A review of mechanisms that impact cost, product quality and patient safety. Journal of Pharmaceutical Science and Research, 8 (5), 271–278.
- Bedogni, G., Garcia, P., Seremeta, K., Okulik, N., Salomon, C. (2023). Preformulation and Long-Term Stability Studies of an Optimized Palatable Praziquantel Ethanol-Free Solution for Pediatric Delivery. Pharmaceutics, 15 (8), 2050. https://doi.org/10.3390/pharmaceutics15082050
- Unahalekhaka, A., Nuthong, P. (2022). Glass particulate adulterated in single dose ampoules: A patient safety concern. Journal of Clinical Nursing, 32 (7-8), 1135–1139. https://doi.org/10.1111/jocn.16336
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Daria Snehyrova, Lyudmila Almakaieva

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






