Development of an emulsion composition with fennel and caraway essential oils for use in the combined therapy of ulcerative colitis
DOI:
https://doi.org/10.15587/2519-4852.2024.302941Keywords:
ulcerative colitis, intestinal disorders, oral microemulsion, Fennel and Caraway essential oilAbstract
The aim of the study is to develop the composition of an emulsion containing essential oils of Fennel and Caraway seeds for use in the symptomatic complex therapy of ulcerative colitis with the aim of eliminating functional intestinal disorders.
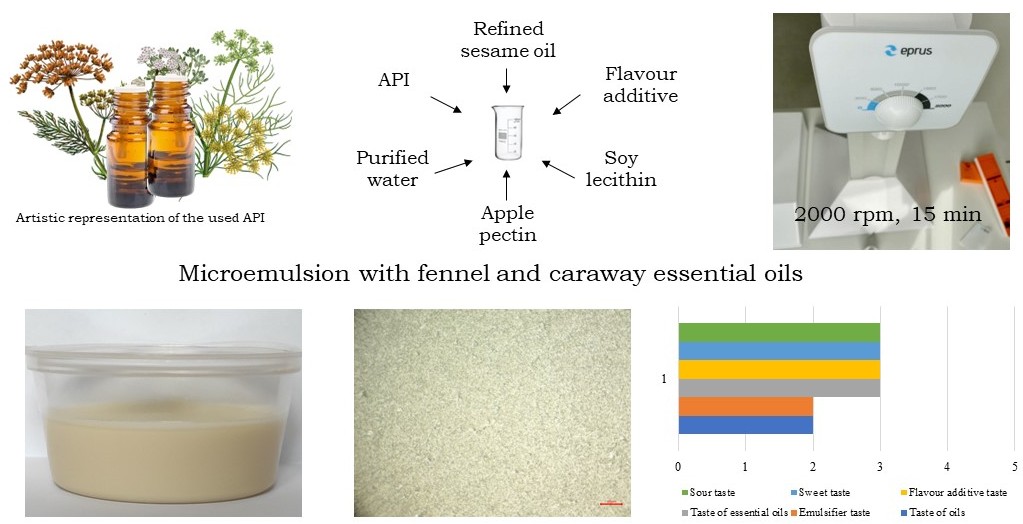
Materials and methods. The objects of the study were samples of emulsions containing active pharmaceutical ingredients (essential oils of Fennel and Caraway), purified water, oils (refined sunflower oil, refined olive oil, refined sesame oil), emulsifiers (polyethylene glycol 40 hydrogenated castor oil, polysorbate 80, polyethylene glycol 100 stearate, acacia gum, guar gum, xanthan gum, soya lecithin), viscosity regulator - apple pectin and flavouring agents (food additives with cherry and tarragon flavour).
Organoleptic properties, stability, rheological parameters, pH, particle size determination by microscopy, and a taste test were carried out with model emulsion samples. Research to establish the optimal technological parameters was carried out in parallel.
Results. The main parameters of the technological process have been established, which allow to obtain an emulsion with evenly distributed particles: 15 minutes at maximum speed. The concentration of emulsifiers at which the emulsions are stable was selected. It was found that the samples containing polyethylene glycol 100 stearate, gums, and soy lecithin have satisfactory organoleptic properties.
The sample with soy lecithin emulsifier differs from the others in its ability to form microemulsions, but it has low viscosity. To improve the rheological properties, apple pectin was added to the emulsion.
The taste test showed that among vegetable oils, refined sesame oil has a more neutral taste, and the flavouring additive "Tarkhun" balances the taste better.
The release of active pharmaceutical ingredients (APIs) from the emulsion base was confirmed by thin-layer chromatography.
Conclusions. A microemulsion with essential oils based on refined sesame oil, soy lecithin, with the addition of viscosity and flavour correctors was developed. The obtained emulsion has satisfactory organoleptic properties and conforms to the requirements for emulsion quality indicators
References
- Horobets, A. O. (2015). Unspecific ulcerative colitis in children. Perinatologiya i pediatriya, 1 (61), 74–80. https://doi.org/10.15574/pp.2015.61.74
- Tomasik, M., Warzyszak, P., Małek, R., Milczek, M., Żołyniak, W., Hawranik, I. et al. (2023). Ulcerative colitis – recent and potential methods of treatment – review. Journal of Education, Health and Sport, 13 (3), 65–73. https://doi.org/10.12775/jehs.2023.13.03.009
- Unifikovanyi klinichnyi protokol pervynnoi, vtorynnoi (spetsializovanoi) ta tretynnoi (vysokospetsializovanoi) medychnoi dopomohy. Zapalni zakhvoriuvannia kyshechnyka (khvoroba krona, vyrazkovyi kolit) (2015). Nakaz Ministerstva okhorony zdorovia Ukrainy No. 90. 11.02.2016. Available at: https://ips.ligazakon.net/document/MOZ26054
- Fylypiuk, O. М., Shmalko, O. O., Vyshnevska, L. I. (2021). Analysis of the assortment of drugs used in functional gastrointestinal disorders at the pharmaceutical market of Ukraine. Social Pharmacy in Health Care, 7 (4), 70–78. https://doi.org/10.24959/sphhcj.21.238
- Abdellaoui, M., Bouhlali, E. dine T., Derouich, M., El-Rhaffari, L. (2020). Essential oil and chemical composition of wild and cultivated fennel (Foeniculum vulgare Mill.): A comparative study. South African Journal of Botany, 135, 93–100. https://doi.org/10.1016/j.sajb.2020.09.004
- Nastanova. Vymohy do vyhotovlennia nesterylnykh likarskykh zasobiv v umovakh aptek. ST-N MOZU 42-4.5:2015. (2015). Kyiv: MOZU. Available at: https://atl.nuph.edu.ua/wp-content/uploads/2022/09/vimogi-do-vigotovlennja-nesterilnih-likarskih-zasobiv-umovah-aptek.pdf
- El-Rady, Mohamed F. A., Rasmy, Nagwa M. H., Yasin, N., Fahmy, H., Amer, A. (2023). Phytochemicals and biological activities of caraway (Carumcarvi L.) essential oil. Egyptian Pharmaceutical Journal, 22 (2), 285–293. https://doi.org/10.4103/epj.epj_154_22
- Aly, A., Maraei, R., Rezk, A., Diab, A. (2022). Phytochemical constitutes and biological activities of essential oil extracted from irradiated caraway seeds (Carum carviL.). International Journal of Radiation Biology, 99 (2), 318–328. https://doi.org/10.1080/09553002.2022.2078004
- Sompendium. Dovidnyk likarskykh preparativ. Available at: https://compendium.com.ua/uk/
- USP Compounding Compendium. The National formulary (2020). Rockville: United States Pharmacopeial Convention, Inc.,
- Sonawane, A., Deshmukh, A., Sonawane, S. (2021). A Review on Self Micro Emulsifying Drug Delivery System (SMEDDS). Ijsrm.Human, 19 (1), 25–33.
- Verma, R. (2023). Development and Evaluation of Polyherbal Formulation with Carminative Effect. Internationale Pharmaceutica Sciencia, 16 (1), 1–8. https://doi.org/10.31531/2231-5896.1000131
- Kabiraj, A., Garai, S., Banerjee, S., Chatterjee, S., Bose, S., Adhikari, A. (2023). A Study of Perception of Safety and Functional Efficacy of a Medicated Digestive Syrup in Gastrointestinal Disorders. The Antiseptic, 120, 23–26.
- Kabiraj, A., Deshmukh, R. (2024). Formulation, Analytical Method Development and Validation of an Emulsion of Multi-enzyme with Carminative Oils. Current Pharmaceutical Analysis, 20 (1), 46–60. https://doi.org/10.2174/0115734129286029240106123114
- Çetin, I., Aydin, M., Bilgiç, Ş. (2024). Matematiksel Modelleme Araştırmalarının İncelenmesi: Betimleyici Bir İçerik Analizi Çalışması. Necatibey Eğitim Fakültesi Elektronik Fen ve Matematik Eğitimi Dergisi, 17 (2), 994–1025. https://doi.org/10.17522/balikesirnef.1321365
- Guidelines for medicine donations (2011). World health organization.
- Derzhavna farmakopeia Ukrainy. Vol. 2 (2015). Kharkiv: DP «Ukrainskyi naukovyi tsentr yakosti likarskykh zasobiv», 723.
- European Pharmacopoeia (2013). European Department for the Quality of Medicines. Strasbourg, 3655.
- The National formulary (2012). Rockville: United States Pharmacopeial Convention, Inc.
- Council of Experts, United States Pharmacopeial Convention (2012). Food Chemicals Codex. Rockville: United States Pharmacopeia (USP).
- DSTU 4765:2007 «Kremy kosmetychni. Zahalni tekhnichni umovy» (2008). Kyiv: Derzhspozhyvstandart Ukrainy.
- Derzhavna farmakopeia Ukrainy. Vol. 1 (2015). Kharkiv: DP «Ukrainskyi naukovyi tsentr yakosti likarskykh zasobiv», 1126.
- Shah, N., Patel, K., Singhvi, I. (2023). Topical Delivery of Eberconazole Nitrate Loaded Microemulsion: Formulation, Design and Evaluation. International Journal of Pharmaceutical Sciences and Drug Research, 15 (4), 478–487. https://doi.org/10.25004/ijpsdr.2023.150412
- Kukhtenko, H., Gladukh, I., Kukhtenko, O., Soldatov, D. (2017). Influence of excipients on the structural and mechanical properties of semisolid dosage forms. Asian Journal of Pharmaceutics, 11 (3), 575–578.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Oleksandr Shmalko, Tetiana Kovalova, Liubov Bodnar, Volodymyr Kovalov, Volodymyr Yakovenko, Liliia Vyshnevska

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






