Study of the chemical components of CO2 extracts from the fruits of Sorbus aucuparia L.
DOI:
https://doi.org/10.15587/2519-4852.2024.303000Keywords:
Sorbus aucuparia L., GC-MS analysis, CO2 extraction, fatty acidAbstract
The article presents the results of the study of the chemical composition of Sorbus aucuparia L. СО2 subcritical extract.
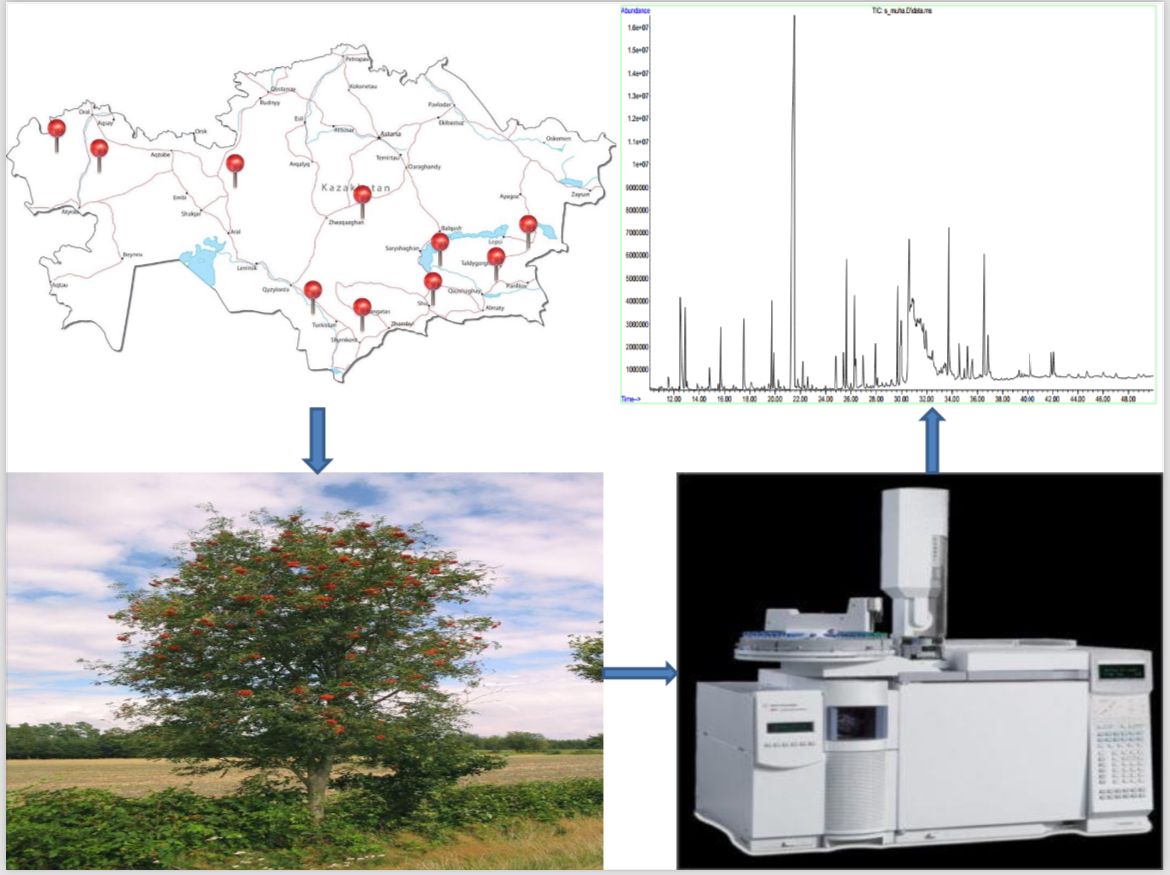
For the first time in Kazakhstan, 20 grams of brown Sorbus aucuparia L. extract were collected using subcritical carbon dioxide extraction. The current study was directed mainly to the chemical compositions of subcritical Sorbus aucuparia L. СО2 subcritical extract.
The Sorbus aucuparia L. extract's chemical compositions were determined using gas chromatography/mass spectrophotometry (GC-MS). The extract included the following main compounds: 5-Methyl-2(3H)-furanone (30.18 %), 5-(3-Ethoxy-4,5-dihydro-isoxazol-5-yl)-5-methyl-imidazolidine-2,4-dione (3.20 %), 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl (2.53 %). Determined fatty acid profile and the moisture content of raw vegetable ingredients of Sorbus substance.
Quantitative determination of fatty acids of ethanol extract was carried out. The results of the analysis for fatty acids in the study showed that linoleic (37.7 %) and oleic (50.5 %) were the most prominent fatty acids.
The aim of this study is to determine the component composition by using the GC-MS method and fatty acid to study the Sorbus aucuparia L. extract obtained by CO2 extraction, which grows in Kazakhstan.
Materials and methods. To determine the possibility of using Sorbus aucuparia L., we carried out the composition and fatty acid of the extract obtained by CO2 extraction in subcritical conditions of Sorbus aucuparia L. by a certain GC-MS method.
Results. The raw materials of the plant were collected in accordance with GACP requirements. Conducted subcritical CO2 extraction of plant raw materials showed a 20 g extraction yield. Chemical compounds were discovered, bioactive components were identified, such as 5-Methyl-2(3H)-furanone (30.18 %), 5-(3-Ethoxy-4,5-dihydro-isoxazol-5-yl)-5-methyl-imidazolidine-2,4-dione (3.20 %), 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl (2.53 %).
Conclusions. The possibility of using the obtained CO2 extract of Sorbus aucuparia L. in the field of pharmaceutical products as a substance and a drug
References
- Grudzinskaya, L., Gemejiyeva, N., Karzhaubekova, Z. (2020). The Kazakhstan medicinal flora survey in a leading families volume. Bulletin of the Karaganda University. “Biology, Medicine, Geography Series,” 100 (4), 39–51. https://doi.org/10.31489/2020bmg4/39-51
- Dickinson, T. A., Lo, E., Talent, N. (2007). Polyploidy, reproductive biology, and Rosaceae: understanding evolution and making classifications. Plant Systematics and Evolution, 266 (1-2), 59–78. https://doi.org/10.1007/s00606-007-0541-2
- Li, M., Ohi-Toma, T., Gao, Y.-D., Xu, B., Zhu, Z.-M., Ju, W.-B., Gao, X.-F. (2017). Molecular phylogenetics and historical biogeography of Sorbus sensu stricto (Rosaceae). Molecular Phylogenetics and Evolution, 111, 76–86. https://doi.org/10.1016/j.ympev.2017.03.018
- Sun, J., Shi, S., Li, J., Yu, J., Wang, L., Yang, X., Guo, L., Zhou, S. (2018). Phylogeny of Maleae (Rosaceae) Based on Multiple Chloroplast Regions: Implications to Genera Circumscription. BioMed Research International, 2018, 1–10. https://doi.org/10.1155/2018/7627191
- Anunciato, T. P., da Rocha Filho, P. A. (2012). Carotenoids and polyphenols in nutricosmetics, nutraceuticals, and cosmeceuticals. Journal of Cosmetic Dermatology, 11 (1), 51–54. https://doi.org/10.1111/j.1473-2165.2011.00600.x
- Sarv, V., Venskutonis, P. R., Bhat, R. (2020). The Sorbus spp. – Underutilised Plants for Foods and Nutraceuticals: Review on Polyphenolic Phytochemicals and Antioxidant Potential. Antioxidants, 9 (9), 813. https://doi.org/10.3390/antiox9090813
- Sarv, V., Venskutonis, P. R., Rätsep, R., Aluvee, A., Kazernavičiūtė, R., Bhat, R. (2021). Antioxidants Characterization of the Fruit, Juice, and Pomace of Sweet Rowanberry (Sorbus aucuparia L.) Cultivated in Estonia. Antioxidants, 10 (11), 1779. https://doi.org/10.3390/antiox10111779
- Zymone, K., Raudone, L., Žvikas, V., Jakštas, V., Janulis, V. (2022). Phytoprofiling of Sorbus L. Inflorescences: A Valuable and Promising Resource for Phenolics. Plants, 11 (24), 3421. https://doi.org/10.3390/plants11243421
- Arvinte, O., Amariei, S. (2022). Сhemical composition of peatland small cranberry (Vaccinium oxycoccus) for potential use as functional ingredient. Ukrainian Food Journal, 11 (3), 416–428. https://doi.org/10.24263/2304-974x-2022-11-3-7
- Rushforth, K. D. (1999). Collins Wildlife Trust Guide: Trees: A Photographic Guide to the Trees of Britain and Europe. New York: HarperCollins, 1336.
- Šedivá, J., Velebil, J., Zahradník, D. (2023). Micropropagation as a Tool for the Conservation of Autochthonous Sorbus Species of Czechia. Plants, 12(3), 488. https://doi.org/10.3390/plants12030488
- Rutkowska, M., Kolodziejczyk-Czepas, J., Owczarek, A., Zakrzewska, A., Magiera, A., Olszewska, M. A. (2021). Novel insight into biological activity and phytochemical composition of Sorbus aucuparia L. fruits: Fractionated extracts as inhibitors of protein glycation and oxidative/nitrative damage of human plasma components. Food Research International, 147, 110526. https://doi.org/10.1016/j.foodres.2021.110526
- Mombekov, S., Orazbekov, Y., Sadykova, N., Kozhamzharova, A., Sharipova, S., Makhatov, Z., Pushkarskaya, N. (2024). Development of antifungal gel, composition and technology based on pomiferin metabolite isolated from fruits of Maclura aurantiaca growing in Kazakhstan. ScienceRise: Pharmaceutical Science, 1 (47), 79–85. https://doi.org/10.15587/2519-4852.2024.299230
- Fan, X.-D., Hou, Y., Huang, X.-X., Qiu, T.-Q., Jiang, J.-G. (2015). Ultrasound-Enhanced Subcritical CO2 Extraction of Lutein from Chlorella pyrenoidosa. Journal of Agricultural and Food Chemistry, 63 (18), 4597–4605. https://doi.org/10.1021/acs.jafc.5b00461
- Hawthorne, S. B., Krieger, M. S., Miller, D. J. (1988). Analysis of flavor and fragrance compounds using supercritical fluid extraction coupled with gas chromatography. Analytical Chemistry, 60 (5), 472–477. https://doi.org/10.1021/ac00156a020
- Bleve, M., Ciurlia, L., Erroi, E., Lionetto, G., Longo, L., Rescio, L. et al. (2008). An innovative method for the purification of anthocyanins from grape skin extracts by using liquid and sub-critical carbon dioxide. Separation and Purification Technology, 64 (2), 192–197. https://doi.org/10.1016/j.seppur.2008.10.012
- Alimzhanova, M. B., Abilev, M. B., Kuandykova, M. M., Kenessov, B. N., Kamysbayev, D. K. (2012). Rapid Screening Method for the Total Petroleum Hydrocarbons in Water Samples by Solid-Phase Microextraction and GC-MS. Eurasian Chemico-Technological Journal, 14 (2), 177. https://doi.org/10.18321/ectj112
- Moldabergenova, A. K., Litvinenko, Yu. A., Akhtayeva, N. Z., Kiekbayeva, L. N., Ross, S. A. (2016). Amino and fatty acid composition of the aerial parts of Еchinops albicaulis, growing in Kazakhstan. International Journal of Biology and Chemistry, 9 (2), 32–35. https://doi.org/10.26577/2218-7979-2016-9-2-32-35
- Grundy, S. M. (1989). Monounsaturated Fatty Acids and Cholesterol Metabolism: Implications for Dietary Recommendations. The Journal of Nutrition, 119 (4), 529–533. https://doi.org/10.1093/jn/119.4.529
- Ramsden, C. E., Ringel, A., Feldstein, A. E., Taha, A. Y., MacIntosh, B. A., Hibbeln, J. R. et al. (2012). Lowering dietary linoleic acid reduces bioactive oxidized linoleic acid metabolites in humans. Prostaglandins, Leukotrienes and Essential Fatty Acids, 87 (4-5), 135–141. https://doi.org/10.1016/j.plefa.2012.08.004
- Karaca, E.; Aytac, S. (2007) The factors affecting on fatty acid composition of oil crops. Anadolu Journal of Agricultural Science, 22, 123–131.
- Nas, S., Gokalp, Y. H., Unsal, M. (2001). Vegetable Oil Technology. Denizli: Pamukkale University Faculty of Architecture Printing House, 322.
- Kantureyeva, A., Ustenova, G., Zvonar Pobirk, A., Mombekov, S., Koilybayeva, M., Amirkhanova, A. et al. (2024). Ceratocarpus arenarius: Botanical Characteristics, Proximate, Mineral Composition, and Cytotoxic Activity. Molecules, 29 (2), 384. https://doi.org/10.3390/molecules29020384
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Serzhan Mombekov, Ubaidilla Datkhayev, Dosmagulova Kalamkul, Assel Kozhamzharova, Ainash Baidullayeva, Mukhamejan Assel, Aigerim Kantureyeva, Zura Yessimsiitova, Damira Yussayeva, Zaure Beken, Akmaral Kydyrkhanova, Aidana Karbozova, Iryna Zhuravel

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






