Pharmacological and technological studies in the development of tablet composition with acorus calamus leaf extract
DOI:
https://doi.org/10.15587/2519-4852.2024.306558Keywords:
Acorus calamus, quercetin, tablets, gastrointestinal tract, relatively therapeutic doseAbstract
The aim: To determine the optimal qualitative and quantitative composition of auxiliary substances for tablets containing dry extract of Acorus calamus leaves and the solid dispersion of quercetin, their relatively therapeutic dose and antiexudative activity.
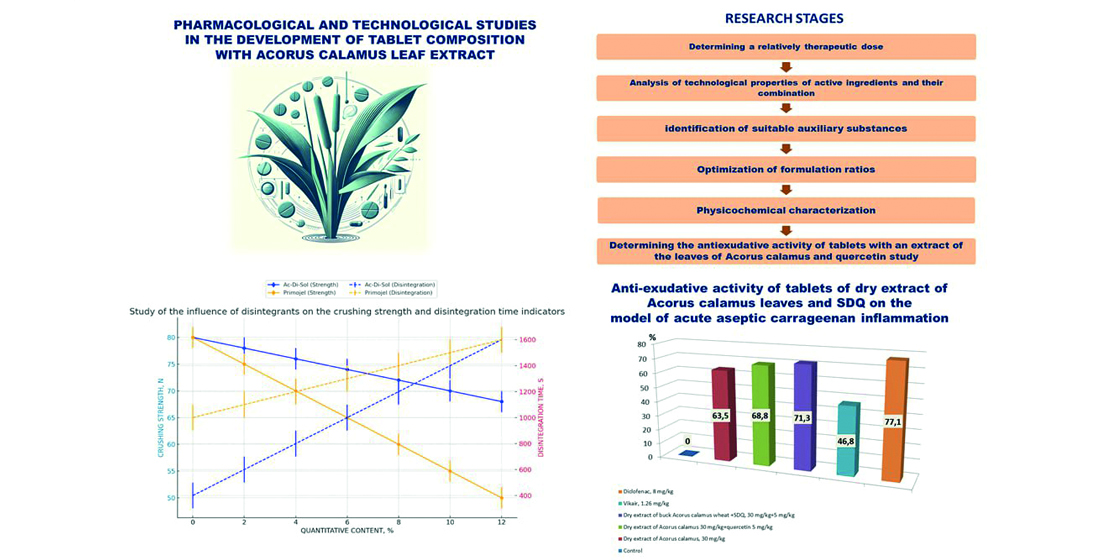
Materials and methods: This study determined a relatively therapeutic dose of dry extract of Acorus calamus leaves, investigated the impact of various auxiliary substances on the properties of tablets formulated with active ingredients - dry extract of Acorus calamus leaves and solid dispersion of quercetin, and assessed antiexudative activity these tablets. The comprehensive analysis entailed the utilization of standardized pharmacopoeial methods to evaluate the quality of the tablet samples. These methods encompassed a range of assessments designed to ensure that the tablets met the requisite pharmacological standards, focusing on key characteristics such as dissolution rate and stability. Determination of the relatively therapeutic dose and antiexudative activity made using standard pharmacological methods in laboratory rats.
Results: In-depth exploration during the study led to identifying Ac-Di-Sol and Lubripharm SSF as the most suitable auxiliary substances for the tablet composition. Detailed analysis revealed that Ac-Di-Sol, when utilized at a 10 % concentration, markedly improved the tablets' disintegration rate without adversely affecting their structural integrity. Concurrently, Lubripharm SSF was observed to significantly enhance the tablets' mechanical stability by reducing their friability.
Conclusions: As a result of the study, the relatively therapeutic dose of dry extract of Acorus calamus leaves, and the solid dispersion of quercetin, optimal auxiliary substances for the tablet formulation - Ac-Di-Sol and Lubripharm SSF - were established. The conducted research enabled the development of a tablet composition that aligns with the requisite pharmacotechnological specifications and conditions of the modern pharmaceutical industry and demonstrates high antiexudative activity relative to monocomponent substances and famous drugs
References
- Wang, Y., Huang, Y., Chase, R. C., Li, T., Ramai, D., Li, S. et al. (2023). Global Burden of Digestive Diseases: A Systematic Analysis of the Global Burden of Diseases Study, 1990 to 2019. Gastroenterology, 165 (3), 773-783.e15. https://doi.org/10.1053/j.gastro.2023.05.050
- Azer, S. A, Awosika, A. O., Akhondi, H. (2023). Gastritis. StatPearls Publishing. Available at: https://www.ncbi.nlm.nih.gov/books/NBK544250/
- Cai, Z., Wang, S., Li, J. (2021). Treatment of Inflammatory Bowel Disease: A Comprehensive Review. Frontiers in Medicine, 8. https://doi.org/10.3389/fmed.2021.765474
- Hall, I. (2022). Gastrointestinal Tract Disorders: Diagnosis and Treatment. New York: Murphy & Moore Publishing, 237.
- Czigle, S., Bittner Fialová, S., Tóth, J., Mučaji, P., Nagy, M. (2022). Treatment of Gastrointestinal Disorders – Plants and Potential Mechanisms of Action of Their Constituents. Molecules, 27 (9), 2881. https://doi.org/10.3390/molecules27092881
- Kelber, O., Bauer, R., Kubelka, W. (2017). Phytotherapy in Functional Gastrointestinal Disorders. Digestive Diseases, 35 (Suppl. 1), 36–42. https://doi.org/10.1159/000485489
- Zhao, Y., Li, J., Cao, G., Zhao, D., Li, G., Zhang, H., Yan, M. (2023). Ethnic, Botanic, Phytochemistry and Pharmacology of the Acorus L. Genus: A Review. Molecules, 28 (20), 7117. https://doi.org/10.3390/molecules28207117
- Iaremenko, M. S., Hontova, T. M. (2017). Porivnialnyi analiz aminokyslotnoho skladu lystia ta korenevyshch lepekhy zvychainoi. Promyslova farmatsiia: Etapy stanovlennia ta maibutnie. Kharkiv: Vyd-vo NFaU, 137–140.
- Derymedvid, L., Korang, L., Shakina, L. (2020). Comparative cytotoxic analysis of extracts obtained from leaves and roots of sweet flag (Acorus Calamus L.) on rat bone marrow cells in vitro. ScienceRise: Pharmaceutical Science, 1 (23), 17–22. https://doi.org/10.15587/2519-4852.2020.196405
- Andryushayev, O., Ruban, O., Maslii, Y., Rusak, I. (2021). Intensification of the extraction process of phenolic compounds from Acorus calamus leaves. ScienceRise: Pharmaceutical Science, 4 (32), 4–10. https://doi.org/10.15587/2519-4852.2021.238329
- Georgiou, N., Kakava, M. G., Routsi, E. A., Petsas, E., Stavridis, N., Freris, C. et al. (2023). Quercetin: A Potential Polydynamic Drug. Molecules, 28 (24), 8141. https://doi.org/10.3390/molecules28248141
- Alizadeh, S. R., Ebrahimzadeh, M. A. (2022). Quercetin derivatives: Drug design, development, and biological activities, a review. European Journal of Medicinal Chemistry, 229, 114068. https://doi.org/10.1016/j.ejmech.2021.114068
- Deepika, Maurya, P. K. (2022). Health Benefits of Quercetin in Age-Related Diseases. Molecules, 27 (8), 2498. https://doi.org/10.3390/molecules27082498
- State Register of Medicinal Products of Ukraine. Available at: http://www.drlz.com.ua
- Regulatory and Directive Documents of Ministry of Health of Ukraine. Available at: https://mozdocs.kiev.ua
- Alkushi, A. G. R., Elsawy, N. A. M. (2017). Quercetin attenuates, indomethacin-induced acute gastric ulcer in rats. Folia Morphologica, 76 (2), 252–261. https://doi.org/10.5603/fm.a2016.0067
- Yuan, K., Zhu, Q., Lu, Q., Jiang, H., Zhu, M., Li, X., Huang, G., Xu, A. (2020). Quercetin alleviates rheumatoid arthritis by inhibiting neutrophil inflammatory activities. The Journal of Nutritional Biochemistry, 84, 108454. https://doi.org/10.1016/j.jnutbio.2020.108454
- Luo, X., Bao, X., Weng, X., Bai, X., Feng, Y., Huang, J. et al. (2022). The protective effect of quercetin on macrophage pyroptosis via TLR2/Myd88/NF-κB and ROS/AMPK pathway. Life Sciences, 291, 120064. https://doi.org/10.1016/j.lfs.2021.120064
- Kovalevska, I. V. (2014). Quercetin physical-chemical characteristics’ definition. Current issues in pharmacy and medicine: science and practice, 1 (14), 9–11.
- Kovalevska, I., Ruban, O., Grudko, V. (2019). Study of biopharmaceutical solubility of quercetin and its solid dispersions. Ukrainian biopharmaceutical journal, 1 (58), 10–16. https://doi.org/10.24959/ubphj.19.209
- Kovalevska, I., Ruban, O., Kutova, O., Levachkova, J. (2021). Optimization of the composition of solid dispersion of quercetin. Current Issues in Pharmacy and Medical Sciences, 34 (1), 1–4. https://doi.org/10.2478/cipms-2021-0001
- Kovalevska, I. V. (2020). Theoretical and experimental substantiation of solid dispersion formation in the development of complex medicinal preparations for the treatment of type II diabetes. [Doctors dissertation; National University of Pharmacy].
- Yaremenko, M., Gontova, T., Boryak, L., Mala, O., Andryushayev, O. (2020). Determination of optimal extraction conditions of phenolic compounds from acorus calamus leaves. EUREKA: Health Sciences, 3, 63–70. https://doi.org/10.21303/2504-5679.2020.001317
- Derzhavna Farmakopeia Ukrainy. Vol. 3 (2018). Kharkiv: Derzhavne pidpryiemstvo «Ukrainskyi naukovyi farmakopeinyi tsentr yakosti likarskykh zasobiv», 732.
- Derzhavna Farmakopeia Ukrainy. Vol. 1 (2015). Kharkiv: Derzhavne pidpryiemstvo «Ukrainskyi naukovyi farmakopeinyi tsentr yakosti likarskykh zasobiv», 1128.
- Stefanova, O. V. (2001). Doklinichni doslidzhennia likarskykh zasobiv. Kyiv Avitsena, 528.
- Herasymets, I., Fira, L., Medvid, I. (2020). Establishment of a conditionally therapeutic dose of dry extract from Reishi Mushrooms on the model of toxic hepatitis. Danish Scientific Journal, 38 (1), 12–16.
- Savych, A. O., Marchyshyn, S. M., Basaraba, R. Yu. (2020). Determination of hypoglycemic activity of the herbal mixtures in screening study. Pharmacology and Drug Toxicology, 14 (5), 344–351. https://doi.org/10.33250/14.05.344
- Yaremenko, M. S., Gontova, T. M., Sira, L. M. (2018). Aboutuse and identification of not officinalis raw materials – Acorus Calamus L. Leaves. Medical and Clinical Chemistry, 1, 105–110. https://doi.org/10.11603/mcch.2410-681x.2018.v0.i1.8772
- Dominici, S., Marescotti, F., Sanmartin, C., Macaluso, M., Taglieri, I., Venturi, F. et al. (2022). Lactose: Characteristics, Food and Drug-Related Applications, and Its Possible Substitutions in Meeting the Needs of People with Lactose Intolerance. Foods, 11 (10), 1486. https://doi.org/10.3390/foods11101486
- Badawy, S. I. F., Shah, K. R., Surapaneni, M. S., Szemraj, M. M., Hussain, M. (2019). Use of Mannitol as a Filler in Wet Granulation. Handbook of Pharmaceutical Wet Granulation, 455–467. https://doi.org/10.1016/b978-0-12-810460-6.00006-3
- Dash, R. P., Srinivas, N. R., Babu, R. J. (2019). Use of sorbitol as pharmaceutical excipient in the present day formulations – issues and challenges for drug absorption and bioavailability. Drug Development and Industrial Pharmacy, 45(9), 1421–1429. https://doi.org/10.1080/03639045.2019.1640722
- Desai, P. M., Liew, C. V., Heng, P. W. S. (2016). Review of Disintegrants and the Disintegration Phenomena. Journal of Pharmaceutical Sciences, 105 (9), 2545–2555. https://doi.org/10.1016/j.xphs.2015.12.019
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Oleksiy Andryushayev, Yevhenii Samoilov, Valeriia Hnatiuk, Olena Ruban, Mariia Velia, Maryna Savokhina

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






