Novel eco-friendly spectrophotometric determination of lercanidipine hydrochloride in tablets using methyl red
DOI:
https://doi.org/10.15587/2519-4852.2024.307263Keywords:
lercanidipine, calcium channel blockers, spectrophotometry, methyl red, validation, quantitative determination, greenness assessment, AGREE, GAPI, pharmacological and technological properties of the tabletsAbstract
The aim of the work was to develop a simple, eco-friendly, quick, affordable and alternative spectrophotometric procedure that uses the azodye methyl red (MR) for the determination of lercanidipine in its dosage form considering the “green” chemistry principles.
Materials and methods. Analytical equipment: Shimadzu UV-1800 double beam UV-visible spectrophotometer (Japan) with included UV-Probe 2.70 software, RAD WAG AS 200/C precise analytical balance (Poland), Elmasonic EASY 40H ultrasonic bath.
Lercanidipine hydrochloride (purity 99 %) was purchased from Jiyan Chemicals (India). Lercanidipine tablets 10 mg and 20 mg were used in our experiments.
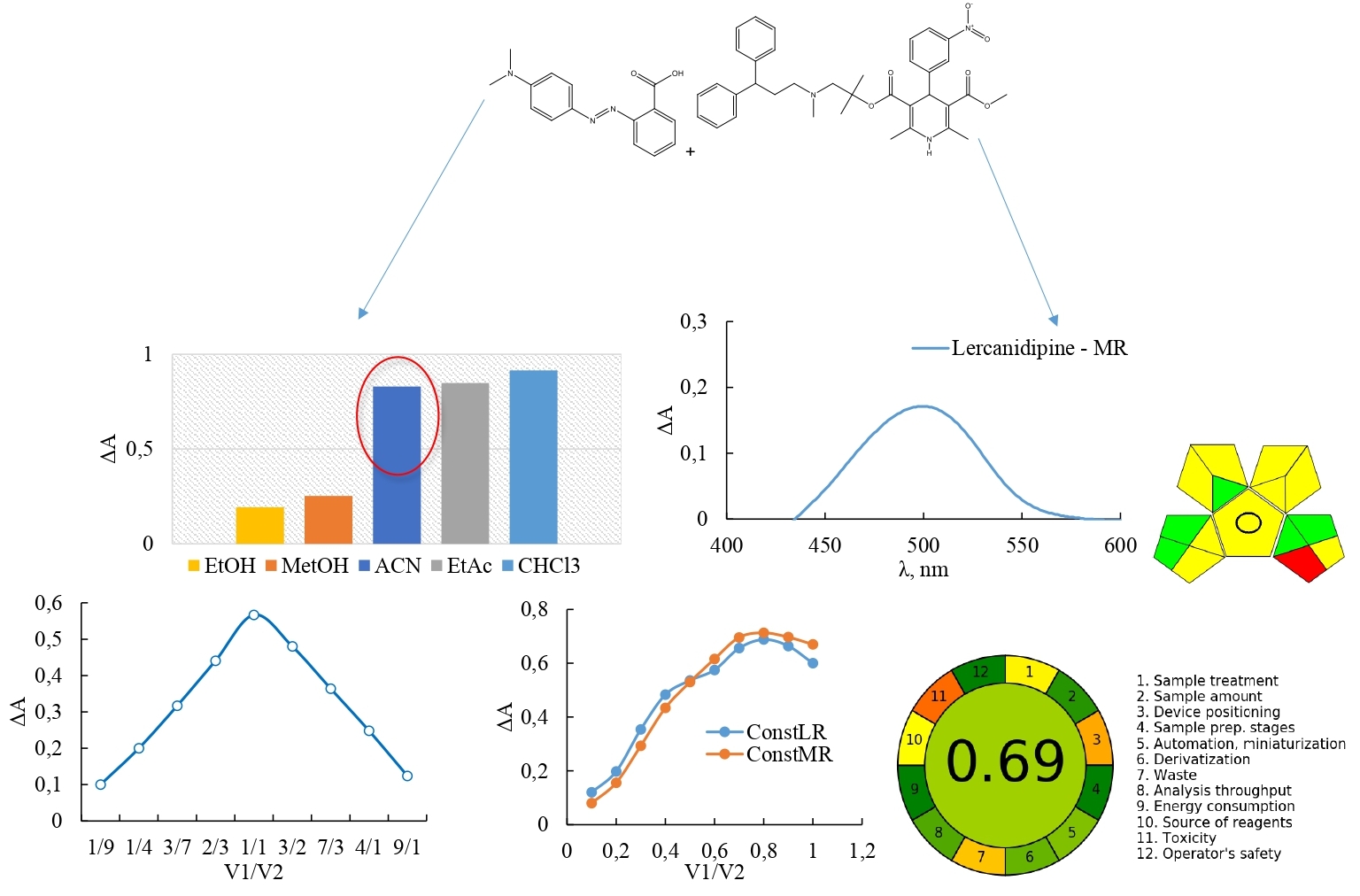
Results and discussion. To determine the amount of lercanidipine in tablets, a spectrophotometric method has been developed. To select the best dye for the method development, we tested a variety of dyes, including MR, bromocresol purple, bromophenol blue, cresol red, bromocresol green and bromothymol blue. We selected MR as the reagent based on the experimental studies' outcomes, and the solvent was an acetonitrile and ethanol mixture with a ratio of 95 to 5. The optimal parameters were determined for the quantitation of lercanidipine in tablets utilizing MR with 5×10-5 mol/L of dye concentration, 0.5 ml of MR solution, at a temperature of 25 °C without heating; detection wavelength was 498 nm and reaction time of 5 min. By using the molar ratios (saturation) method and Job's (continuous variations) approach, the stoichiometric coefficients of reacting components involving lercanidipine and dye were established to be 1:1. The proposed spectrophotometric procedure was linear within the concentration ranging from 6.48 – 32.41 μg/mL. Using the least squares method, a regression equation was generated: y = 0.0208x – 0.0318. The correlation coefficient was higher than 0.999, indicating that the analytical procedures' linearity is acceptable; the limit of detection and limit of quantitation were 1.19 μg/mL and 3.62 μg/mL, respectively. The robustness, accuracy and precision of the study results fell within acceptable limits. The proposed method was successfully applied to determine the content of lercanidipine in its tablet dosage forms. The analysis of the method's "greenness" using AGREE and GAPI tools yielded excellent results.
Conclusions. The method that has been developed can serve as an alternative approach for the routine control of lercanidipine content in its tablets
References
- Grassi, G., Robles, N. R., Seravalle, G., Fici, F. (2017). Lercanidipine in the Management of Hypertension: An Update. Journal of Pharmacology and Pharmacotherapeutics, 8 (4), 155–165. https://doi.org/10.4103/jpp.jpp_34_17
- Lercanidipine. PubChem Available at: https://pubchem.ncbi.nlm.nih.gov/compound/Lercanidipine
- European Pharmacopoeia. 11 ed. (2022). Available at: https://www.edqm.eu/en/european-pharmacopoeia-ph.-eur.-11th-edition
- El-Masry, A. A., El-Wasseef, D. R., Eid, M., Shehata, I. A., Zeid, A. M. (2022). Development of three ecological spectroscopic methods for analysis of betrixaban either alone or in mixture with lercanidipine: greenness assessment. Royal Society Open Science, 9 (2). https://doi.org/10.1098/rsos.211457
- Manikya, T. S., Sujana, U. K., Nagalakshmi, K. V. (2017). Assay of bioactive compound: lercanidipine hydrochloride by oxidative coupling reactions in dosage forms. International Journal of Advance Research in Science and Engineering, 6 (8), 409–416. Available at: http://ijarse.com/images/fullpdf/1502607563_ijarse164.pdf
- Lobhe, G. A., Grampurohit, N. D., Dhobale, S. M., Gaikawad, D. D. (2013). Application of planar chromatography for estimation of lercanidipine hydrochloride in dosage form. Journal of Pharmacy Research, 6 (1), 129–133. https://doi.org/10.1016/j.jopr.2012.11.027
- Wankhede, S. B., Philip, S. M. (2016). Sensitive high performance thin layer chromatographic determination of lercanidipine hydrochloride in pharmaceuticals and in blood plasma. Eurasian Journal of Analytical Chemistry, 11, 141–154.
- El-Masry, A. A., El-Wasseef, D. R., Eid, M., Shehata, I. A., Zeid, A. M. (2021). Optimization and Validation of a Facile RP-HPLC Method for Determination of Betrixaban and Lercanidipine in Pharmaceutical and Biological Matrices. Journal of Chromatographic Science, 59 (8), 785–794. https://doi.org/10.1093/chromsci/bmab088
- Udupa, N., Chonkar, A., Managuli, R., Rao, J. (2016). Development and validation of reversed phase high-performance liquid chromatography method for estimation of lercanidipine HCl in pure form and from nanosuspension formulation. Journal of Basic and Clinical Pharmacy, 7 (1), 17–22. https://doi.org/10.4103/0976-0105.170586
- Li, X., Shi, F., He, X., Jian, L., Ding, L. (2016). A rapid and sensitive LC–MS/MS method for determination of lercanidipine in human plasma and its application in a bioequivalence study in Chinese healthy volunteers. Journal of Pharmaceutical and Biomedical Analysis, 128, 67–72. https://doi.org/10.1016/j.jpba.2016.05.013
- Sabi-mouka, E. M. B., Agbokponto, J. E., Zhang, R., Li, Q., Ding, L. (2016). Simultaneous Determination of a Fixed-Dose Combination of Lercanidipine and Valsartan in Human Plasma by LC–MS-MS: Application to a Pharmacokinetic Study. Journal of Chromatographic Science, 54 (9), 1553–1559. https://doi.org/10.1093/chromsci/bmw102
- Chaudhary, D. V., Patel, D. P., Shah, P. A., Shah, J. V., Sanyal, M., Shrivastav, P. S. (2016). Determination of lercanidipine in human plasma by an improved UPLC–MS/MS method for a bioequivalence study. Journal of Pharmaceutical Analysis, 6 (2), 87–94. https://doi.org/10.1016/j.jpha.2015.09.001
- Lourenço, L. P., Aguiar, F. A., de Oliveira, A. R. M., de Gaitani, C. M. (2015). Quantitative Determination of Lercanidipine Enantiomers in Commercial Formulations by Capillary Electrophoresis. Journal of Analytical Methods in Chemistry, 2015, 1–9. https://doi.org/10.1155/2015/294270
- Altun, Y., Uslu, B., Ozkan, S. A. (2010). Electroanalytical Characteristics of Lercanidipine and its Voltammetric Determination in Pharmaceuticals and Human Serum on Boron-Doped Diamond Electrode. Analytical Letters, 43 (12), 1958–1975. https://doi.org/10.1080/00032711003687047
- Halka, L., Kucher, T., Kryskiw, L., Piponsk, M., Furdela, I., Uglyar, T. et al. (2023). Development of the spectrophotometric method for the determination of rosuvastatin in tablets by using bromophenol blue. ScienceRise: Pharmaceutical Science, 2 (42), 11–19. https://doi.org/10.15587/2519-4852.2023.277461
- Peleshok, K., Bondar, B., Kryskiw, L., Kucher, T., Poliak, O., Logoyda, L. (2021). Non-extractive spectrophotometric determination of valsartan in pure form and in pharmaceutical products by ion-pair complex formation with bromophenol blue and methyl red. Pharmacia, 68 (4), 851–858. https://doi.org/10.3897/pharmacia.68.e73559
- Lercanidipine. Cayman. Available at: https://cdn.caymanchem.com/cdn/insert/29104.pdf
- Methyl red. PubChem. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/10303
- Green Solvent Selection Tool. Available at: https://green-solvent-tool.herokuapp.com/
- State Pharmacopoeia of Ukraine. Vol. 1 (2015). Kharkiv: SE “Ukrainian Scientific Pharmacopoeial Center for Quality of Medicines”, 11148
- Ermer, J., Agut, C. (2014). Precision of the reportable result. Simultaneous optimisation of number of preparations and injections for sample and reference standard in quantitative liquid chromatography. Journal of Chromatography A, 1353, 71–77. https://doi.org/10.1016/j.chroma.2014.03.043
- Pena-Pereira, F., Wojnowski, W., Tobiszewski, M. (2020). AGREE – Analytical GREEnness Metric Approach and Software. Analytical Chemistry, 92 (14), 10076–10082. https://doi.org/10.1021/acs.analchem.0c01887
- Płotka-Wasylka, J. (2018). A new tool for the evaluation of the analytical procedure: Green Analytical Procedure Index. Talanta, 181, 204–209. https://doi.org/10.1016/j.talanta.2018.01.013
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Liubomyr Kryskiw, Mariana Horyn, Tetyana Kucher, Nadiya Zarivna, Olha Poliak, Liliya Logoyda

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.







