Full green assay of rosuvastatin utilizing sulphophtalein dyes: application to tablet analysis
DOI:
https://doi.org/10.15587/2519-4852.2024.310564Keywords:
Rosuvastatin, Spectrophotometry, Sulphophtalein dyes, Bromocresol green, Bromocresol purple, Bromothymol blue, Tablet, ValidationAbstract
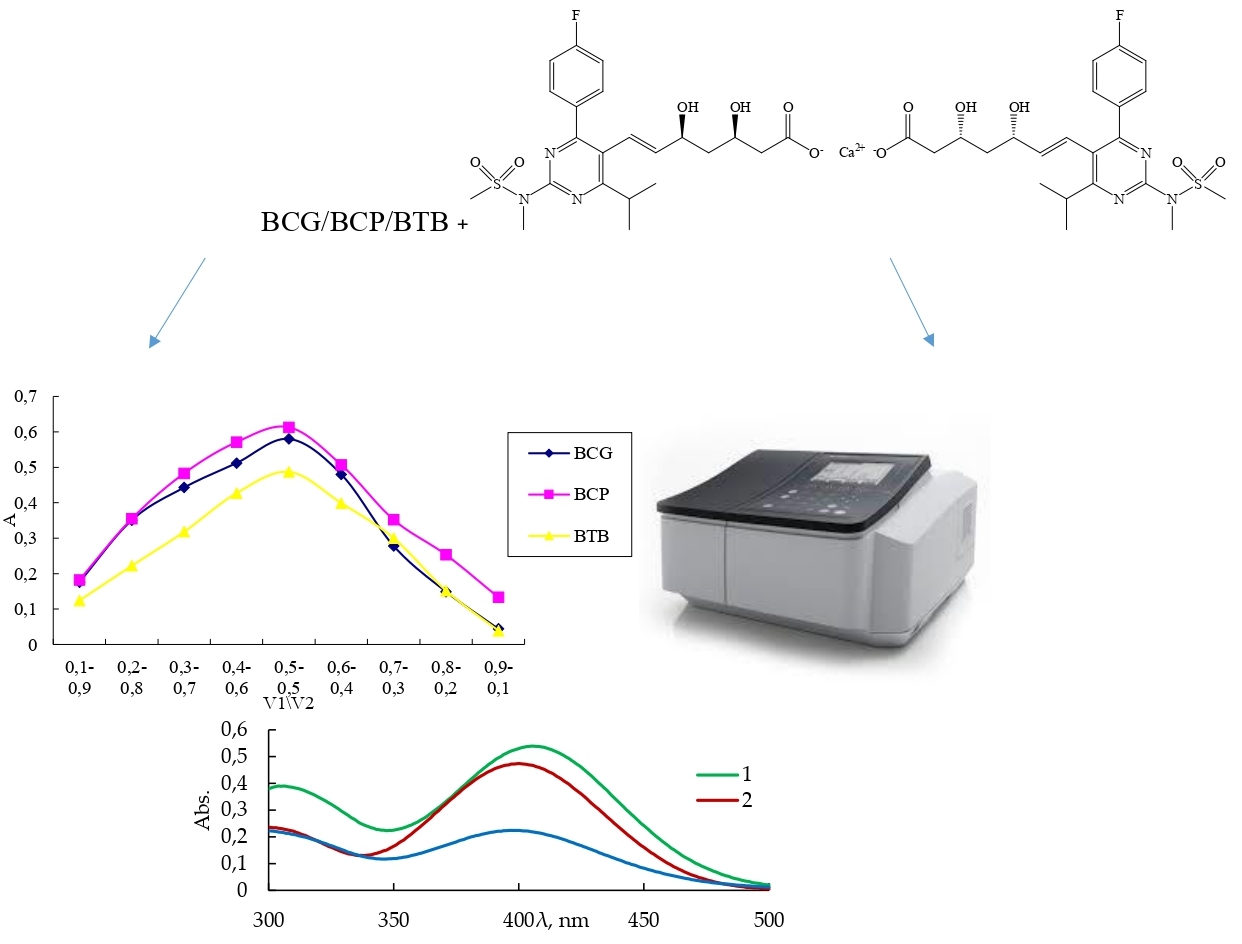
The aim of the work was to develop a «green» and extraction-free spectrophotometric procedure for the assay of rosuvastatin in tablets. The present work describes three new spectrophotometric procedures (A, B and C) that can be utilized for routine quality control of rosuvastatin in laboratories.
Materials and methods. Analytical instrumentation: Shimadzu UV-1800 double beam UV-vis spectrophotometer (Japan) with attached UV-Probe ver. 2.62 software, RAD WAG AS 200/C precise analytical balance (Poland). Rosuvastatin calcium (purity ≥98 % (HPLC)) was bought from Sigma-Aldrich Chemicals Co. (St. Louis, MO, USA). Rosuvastatin 10 mg tablets were acquired from a nearby drugstore. All solvents used in this study, including methanol, ethanol, chloroform, acetonitrile and ethyl acetate, were produced by Honeywell and had a purity of 99.9 %. BCG, BCP and BTB were acquired from Sigma-Aldrich Chemicals Co. (USA, St. Louis). All chemicals utilized in the experiment were of analytical purity.
Results and discussion. New simple «green» and extraction-free spectrophotometric procedures for assay of rosuvastatin in tablets involve the formation of ion-pair complexes with sulphophtalein dyes (BCG (Method A), BCP (Method B), BTB (Method C)) have been developed. The absorbances of the coloured reaction products were registered at 405 nm (Method A) and 400 nm (Methods B, C). The concentration was linearly proportional to absorbance values in the range of 2.51-20.08 μg/mL (method A), 2.50-24.90 μg/mL (method B) and 2.51-12.56 μg/mL (method C). Estimation of LOD and LOQ parameters were obtained as 0.67 μg/mL and 2.23 μg/mL (Method A), 0.39 μg/mL and 1.32 μg/mL (Method B), 0.30 μg/mL and 1.01 μg/mL (Method C). The stoichiometric ratio of the reactive components of rosuvastatin - BCG, BCP, and BTB corresponded 1: 1. The %RSD values of intra-day and inter-day were obtained less than < 1.5 %, which showed excellent repeatability and RE % data was ≤ 3 %. The effect on the environment of the proposed spectrophotometric procedures and their compliance with the GAC principles were endorsed by the output of AGREE, GAPI, and AES metrics tools. The values of these three «green» metrics show that the proposed spectrophotometric procedures had a low environmental impact compared with the reported ones.
Conclusions. The developed fast, simple and cost-effective methods A, B, and C can be used for routine analysis of rosuvastatin in tablets
References
- Rosuvastatin. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/Rosuvastatin#section=Vapor-Pressure Last accessed: 22.01.2024
- Smith, G., Davidson, R., Bloor, S., Burns, K., Calnan, C., McAulay, P., Torr, N., Ward, W., McTaggart, F. (2000). Pharmacological properties of ZD4522 – A new HMG-CoA reductase inhibitor. Atherosclerosis, 151 (1), 39. https://doi.org/10.1016/s0021-9150(00)80176-8
- European Pharmacopoeia. 11ed. (2022). Available at: https://www.edqm.eu/en/european-pharmacopoeia-ph.-eur.-11th-edition(accessed on 22 October 2023).
- Ângelo, M. L., Moreira, F. de L., Morais Ruela, A. L., Santos, A. L. A., Salgado, H. R. N., de Araújo, M. B. (2018). Analytical Methods for the Determination of Rosuvastatin in Pharmaceutical Formulations and Biological Fluids: A Critical Review. Critical Reviews in Analytical Chemistry, 48 (4), 317–329. https://doi.org/10.1080/10408347.2018.1439364
- Gupta, A., Mishra, P., Shah, K. (2008). Simple UV Spectrophotometric Determination of Rosuvastatin Calcium in Pure Form and in Pharmaceutical Formulations. Journal of Chemistry, 6 (1), 89–92. https://doi.org/10.1155/2009/956712
- Sevda, R., Ravetkar, A., Shirote, P. (2011). UV Spectrophotometric estimation of rosuvastatin calcium and fenofibrate in bulk drug and dosage form using simultaneous equation method. International Journal of ChemTech Research, 3 (2), 629–635.
- Parmar, V., Solanki, H., Prajapati L. (2013). Derivative spectrophotometric determination of rosuvastatin calcium and fenofibrate in tablet dosage form. Inventi Rapid: Pharm Analysis & Quality Assurance, 2, 1–5.
- Uyar, B., Celebier, M., Altinoz, S. (2007). Spectrophotometric determination of rosuvastatin calcium in tablets. Die Pharmazie, 62 (6), 411–413.
- Afroz, A., Haque, T., Talukder, M., Islam, S. (2011). Spectrophotometric estimation of rosuvastatin calcium and glimepiride in tablet dosage form. Asian Journal of Pharmaceutical Analysis, 1 (4), 74–78.
- Krishna, M. V., Sankar, D. G. (2006). Extractive Spectrophotometric Methods for the Determination of Rosuvastatin Calcium in Pure Form and in Pharmaceutical Formulations by Using Safranin O and Methylene blue. Journal of Chemistry, 4 (1), 46–49. https://doi.org/10.1155/2007/454853
- Ramadan, A., Mandil, H., Alshelhawi, N. (2014). Spectrophotometric determination of rosuvastatin calcium in pure form and pharmaceutical formulations by the oxidation using iodine and formation triiodide complex in acetonitrile. International Journal of Pharmacy and Pharmaceutical Sciences, 6 (5), 579–585.
- Lima, M. F., Cassella, R. J., Pacheco, W. F. (2017). Spectrophotometric determination of rosuvastatin in pharmaceutical formulations using quinalizarin. Brazilian Journal of Pharmaceutical Sciences, 53 (3). https://doi.org/10.1590/s2175-97902017000300075
- Ramadan, A., Mandil, H., Alsayed-Ali, R. (2015). Spectrophotometric determination of rosuvastatin in pure form and pharmaceutical formulations through ion-pair complex formation using bromocresol green. International Journal of Pharmacy and Pharmaceutical Sciences, 7 (11), 191–198.
- Prajapati, P., Bodiwala, K., Marolia, B., Rathod, I., Shah, S. (2010). Development and validation of extractive spectrophotometric method for determination of rosuvastatin calcium in pharmaceutical dosage forms. Journal of Research in Pharmacy, 3 (8), 2036–2038.
- Rao, G., Shaiba, M., Bhargavi, P., Kumar, T., Swethapriya, C. H. B. (2010). Spectrophotometric methods for the determination of rosuvastatin. Oriental Journal of Chemistry, 3, 1215–1217.
- Halka, L., Kucher, T., Kryskiw, L., Piponsk, M., Furdela, I., Uglyar, T. et al. (2023). Development of the spectrophotometric method for the determination of rosuvastatin in tablets by using bromophenol blue. ScienceRise: Pharmaceutical Science, 2 (42), 11–19. https://doi.org/10.15587/2519-4852.2023.277461
- Karunakaran, A., Subhash, V., Chinthala, R., Muthuvijayan, J. (2011). Simultaneous Estimation of Rosuvastatin Calcium and Fenofibrate in Bulk and in Tablet Dosage Form by UV-Spectrophotometry and RP-HPLC. Stamford Journal of Pharmaceutical Sciences, 4 (1), 58–63. https://doi.org/10.3329/sjps.v4i1.8868
- Sharma, S., Bhandari, P. (2005). Simultaneous Estimation of Rosuvastatin Calcium and Fenofibrate in Bulk and in Tablet Dosage Form by UV-Spectrophotometry and RP-HPLC. J. Pharm. Res, 5, 2311–2314.
- ICH Validation of Analytical Procedures: Text and Methodology, Q2 (R1) (2005). Geneva. Available et: https://www.ich.org/page/quality-guidelines Last accessed: 24.01.2024
- Gałuszka, A., Konieczka, P., Migaszewski, Z., Namies’nik, J. (2012). Analytical eco-scale for assessing the greenness of analytical procedures. TrAC Trends in Analytical Chemistry, 37, 61–72. https://doi.org/10.1016/j.trac.2012.03.013
- Płotka-Wasylka, J. (2018). A new tool for the evaluation of the analytical procedure: Green Analytical Procedure Index. Talanta, 181, 204–209. https://doi.org/10.1016/j.talanta.2018.01.013
- Pena-Pereira, F., Wojnowski, W., Tobiszewski, M. (2020). AGREE – Analytical GREEnness Metric Approach and Software. Analytical Chemistry, 92 (14), 10076–10082. https://doi.org/10.1021/acs.analchem.0c01887
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Liudmyla Halka, Tetyana Kucher, Liubomyr Kryskiw, Marjan Piponski, Mariana Horyn, Olha Poliak, Nadiya Zarivna, Liliya Logoyda

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






