Ultra-performance liquid chromatography-mass spectrometry methods for the determination of the residual quantities of ramipril and hydrochlorothiazide for controlling the cleaning of equipment
DOI:
https://doi.org/10.15587/2519-4852.2024.310759Keywords:
hydrochlorothiazide, ramipril, equipment cleaning control, validation, UPLCAbstract
Monitoring the completeness of equipment cleaning is essential to prevent cross-contamination of medicinal products. Therefore, it is necessary to develop fast and sensitive methods for studying residual quantities of active ingredients on the surfaces of technological equipment.
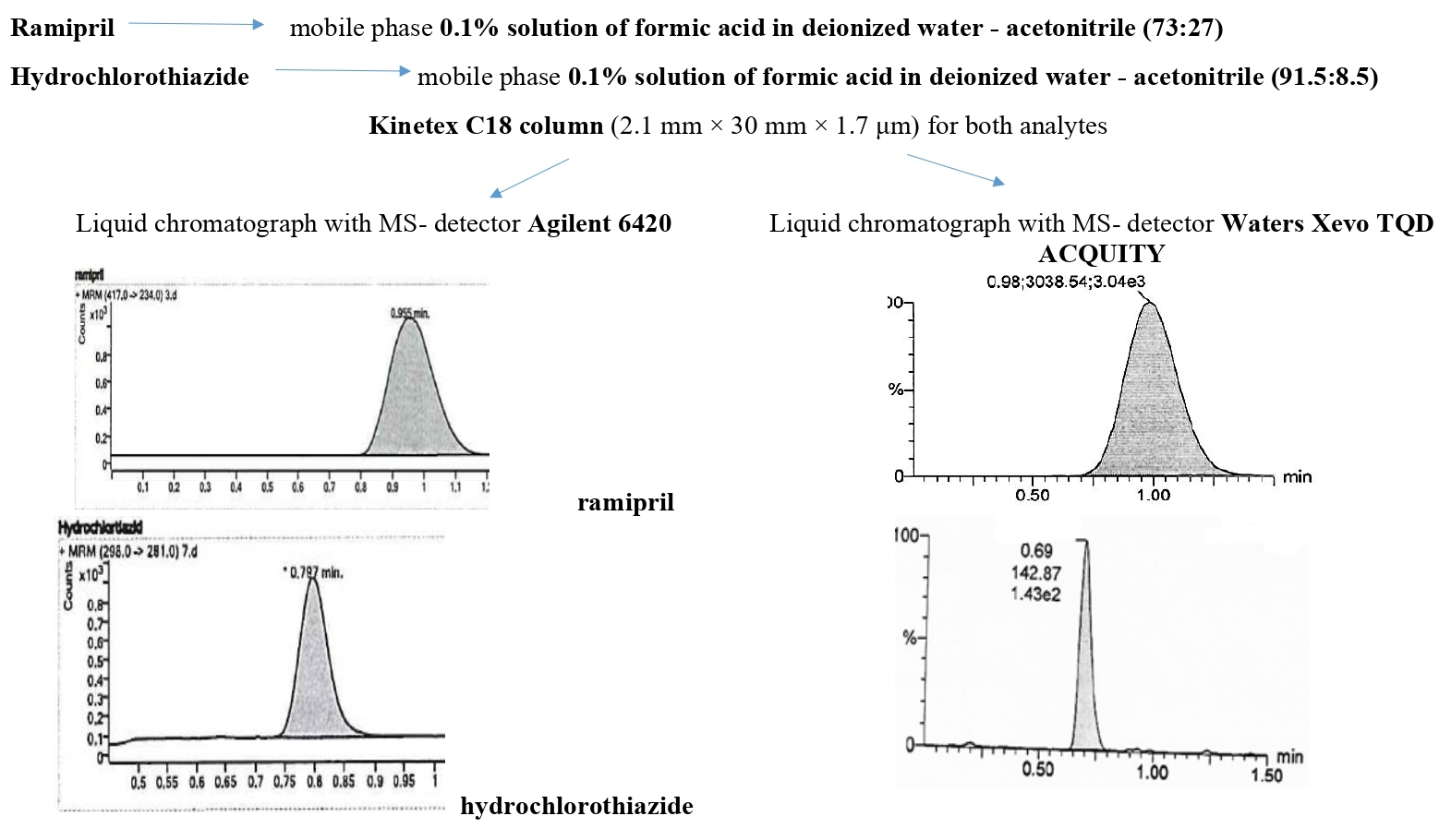
The aim of the work was to develop and validate analytical methods for the determination of ramipril and hydrochlorothiazide in wash waters by ultra-performance liquid chromatography–mass spectrometry method.
Materials and methods. In the study, standard samples of ramipril (USP RS) and hydrochlorothiazide (USP RS), as well as class A reagents, were used. Samples were analysed on a liquid chromatograph with an MS detector (Agilent 6420 and Waters Xevo TQD ACQUITY). We used the Kinetex C18 column (2.1 mm × 30 mm × 1.7 μm); mobile phase – 0.1% formic acid solution in deionised water – Acetonitrile (ratio 73:27 for the determination of ramipril and 91.5:8.5 for the determination of hydrochlorothiazide); mobile phase rate of 0.4 mL/min for the determination of ramipril and 0.35 mL/min for the determination of hydrochlorothiazide; column temperature 45 °C for the determination of ramipril and 40 °C for the determination of hydrochlorothiazide, ionisation mode - electric spray in positive mode; The detection parameters are the mode of registration of the daughter ion 417 → 234 m/z for the determination of ramipril and 298 → 281 m/z for the determination of hydrochlorothiazide.
Results and discussion. Methods for the determination of ramipril and hydrochlorothiazide in wash waters by ultra-performance liquid chromatography–mass spectrometry have been developed. The developed methods have sufficient linearity, correctness and precision. The sensitivity of the techniques was confirmed at the level of 0.0026 μg/ml. The techniques can be used in the concentration range of 0.0026 – 0.0255 μg/ml
Conclusions. Analytical methods for determining ramipril and hydrochlorothiazide in wash waters have been developed and validated.
References
- Good Manufacturing Practice (GMP) guidelines (EudraLex – Volume 4). Available at: https://health.ec.europa.eu/medicinal-products/eudralex/eudralex-volume-4_en Last accessed: 06.06.2024
- Resto, W., Hernández, D., Rey, R., Colón, H., Zayas, J. (2007). Cleaning validation 2: Development and validation of an ion chromatographic method for the detection of traces of CIP-100 detergent. Journal of Pharmaceutical and Biomedical Analysis, 44 (1), 265–269. https://doi.org/10.1016/j.jpba.2007.01.037
- Bubnič, Z., Urleb, U., Kreft, K., Veber, M. (2010). The application of atomic absorption spectrometry for the determination of residual active pharmaceutical ingredients in cleaning validation samples. Drug Development and Industrial Pharmacy, 37 (3), 281–289. https://doi.org/10.3109/03639045.2010.509726
- Kolodsick, K. J., Phillips, H., Feng, J., Kingsmill, C. A. (2006). Enhancing Drug development by Applying LC-MS-MS for Cleaning Validation in Manufacturing Equipment. Pharmaceutical Technology, 30 (2), 56–72.
- Heidbreder, D., Froer, K.L., Bauer, B., Cairns, V., Breitstadt, A. (1991). Efficacy and Safety of Ramipril in Combination with Hydrochlorothiazide Results of a Long-Term Study. Journal of Cardiovascular Pharmacology, 18, 169–173. https://doi.org/10.1097/00005344-199100182-00039
- Lakshmi, K. S., Sivasubramanian, L. (2010). A stability indicating hplc method for the simultaneous determination of valsartan and ramipril in binary combination. Journal of the Chilean Chemical Society, 55 (2), 223–226. https://doi.org/10.4067/s0717-97072010000200017
- Bhagwate, S., Gaikwad, N. (2013). Stability indicating HPLC method for the determination of hydrochlorothiazide in pharmaceutical dosage form. Journal of Applied Pharmaceutical Science, 3, 88–92. https://doi.org/10.7324/japs.2013.30215
- Szpot, P., Buszewicz, G. (2015). Determination of ramipril in human plasma and its fragmentation by UPLC-Q-TOF-MS with positive electrospray ionization. Acta Pharmaceutica, 65 (2), 159–169. https://doi.org/10.1515/acph-2015-0018
- Babu, K. A., Kumar, G. V., Sivasubramanian, L. (2011). Simultaneous estimation of ramipril and amlodipine in pharmaceutical dosage form by RP-HPLC method. International Journal of Pharmacy and Pharmaceutical Sciences, 3 (4), 196–198.
- Dai, S.-Y., Qiu, S.-T., Wu, W., Fu, C.-M. (2013). Development and validation of an rp-hplc method for simultaneous determination of Ramipril and Amlodipine in tablets. Journal of Pharmaceutical Analysis, 3 (6), 440–446. https://doi.org/10.1016/j.jpha.2013.09.002
- Elshanawane, A. A., Mostafa, S. M., Elgawish, M. S. (2008). Application of a Validated, Stability-Indicating LC Method to Stress Degradation Studies of Ramipril and Moexipril.HCl. Chromatographia, 67 (7-8), 567–573. https://doi.org/10.1365/s10337-008-0544-3
- Gupta, K. R., Wankhede, S. B., Tajne, M. R., Wadodkar, S. G. (2007). Simultaneous determination of Amlodipine and Ramipril by high performance thin layer chromatography. Asian Journal of Chemistry, 19, 4177–4182.
- Logoyda, L. (2019). Analysis of approaches to the development and validation of the methods of analysis of some active pharmaceutical ingredients from the group of angiotensin converting enzyme inhibitors in drugs and biological liquids. International Journal of Applied Pharmaceutics, 11 (4). https://doi.org/10.22159/ijap.2019v11i4.32420
- Kumar, A. M., Kumar, P. V., Nasare, M., Rao, V., Parasad, V. V. L., Diwan, V. P. (2012). Isocratic RP-HPLC estimation of Ramipril and Amlodipine in pharmaceutical dosage form. Journal of Advanced Pharmacy Education and Research, 2, 137–145.
- Maste, M. M., Kalekar, M. C., Kadian, N., Bhat, A. R. (2011). Development and validation of RP-HPLC method for simultaneous estimation of Amlodipine and Ramipril in bulk and tablet dosage form. Asian Journal of Research in Chemistry, 4, 1210–1213.
- Panchal, H. J., Suhagia, B. N., Patel, N. J., Rathod, I. S., Patel, B. H. (2008). Simultaneous Estimation of Atorvastatin Calcium, Ramipril and Aspirin in Capsule Dosage Form by RP-LC. Chromatographia, 69 (1-2), 91–95. https://doi.org/10.1365/s10337-008-0831-z
- Patel, J., Patel, M. (2014). RP-HPLC method development and validation for the simultaneous estimation of ramipril and amlodipine besylate in capsule dosage form. Journal of Chemical and Pharmaceutical Research, 6, 725–733.
- Patole, S. M., Khodke, A. S., Potale, L. V., Damle, M. C. (2010). A validated HPLC method for analysis of atorvastatin calcium, ramipril and asprin as the bulk drug and in combined capsule dosage forms. International Journal of Pharmaceutical Sciences Review and Research, 4, 40–45.
- Rajput, P. S., Kaur, A., Gill, N. K., Mittal, K., Sarma, G. S. (2012). Simultaneous estimation of ramipril and amlodipine in bulk and tablet dosage form by RP-HPLC method. Journal of Applied Pharmaceutical Science, 2 (7), 160–165. https://doi.org/10.7324/japs.2012.2724
- Dheeravath, S. N., Ramadevi, K., Saraswathi, Z., Maniklal, D., Bhagawan. D, Bhagawan. D. (2012). RP-HPLC method development for simultaneous determination of the drugs Ramipril and Amlodipine. International Journal of Scientific Research, 2 (2), 364–367. https://doi.org/10.15373/22778179/feb2013/123
- Sharma, R., Khanna, S., Mishra, G. P. (2011). Development and Validation of RP‐HPLC Method for Simultaneous Estimation of Ramipril, Aspirin and Atorvastatin in Pharmaceutical Preparations. Journal of Chemistry, 9 (4), 2177–2184. Portico. https://doi.org/10.1155/2012/891695
- Rao, S., Srinivas, K. (2010). RP-HPLC method for the determination of losartan potassium and ramipril in combined dosage form. Indian Journal of Pharmaceutical Sciences, 72 (1), 108–111. https://doi.org/10.4103/0250-474x.62243
- Lincy, J., Mathew, G., Venkata, R. (2018). Simultaneous estimation of Atorvastatin and Ramipril by RP-HPLC and spectroscopy. Pakistan Journal of Pharmaceutical Sciences, 21, 282–284.
- Sharma, A., Shah, B., Patel, B. (2010). Scholars Research Library Simultaneous Estimation of Atorvastatin Calcium, Ramipril and Aspirin in Capsule Dosage Form Using HPTLC. Der Pharma Chem., 2, 10–16.
- Żuromska-Witek, B., Stolarczyk, M., Szlósarczyk, M., Kielar, S., Hubicka, U. (2022). Simple, Accurate and Multianalyte Determination of Thirteen Active Pharmaceutical Ingredients in Polypills by HPLC-DAD. Chemosensors, 11 (1), 25. https://doi.org/10.3390/chemosensors11010025
- De Diego, M., Godoy, G., Mennickent, S., Olivares, M., Godoy, R. (2010). Stress degradation studies of ramipril by a validated stability-indicating liquid chromatographic method. Journal of the Chilean Chemical Society, 55 (4), 450–453. https://doi.org/10.4067/s0717-97072010000400008
- Typlynska, K., Kondratova, Y., Logoyda, L. (2023). Development of Methods of Quality Control of the Tablets «Ramipril». Scientia Pharmaceutica, 91 (2), 21. https://doi.org/10.3390/scipharm91020021
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Kateryna Typlynska, Yuliya Kondratova, Mariana Horyn, Liliya Logoyda

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






