Microencapsulation for the delivery of terazosin hydrochloride: design, development, and characterization
DOI:
https://doi.org/10.15587/2519-4852.2024.310813Keywords:
Terazosin, sustained release, microparticles, HPLC method, Validation, Kolliwax, Zeta potentialAbstract
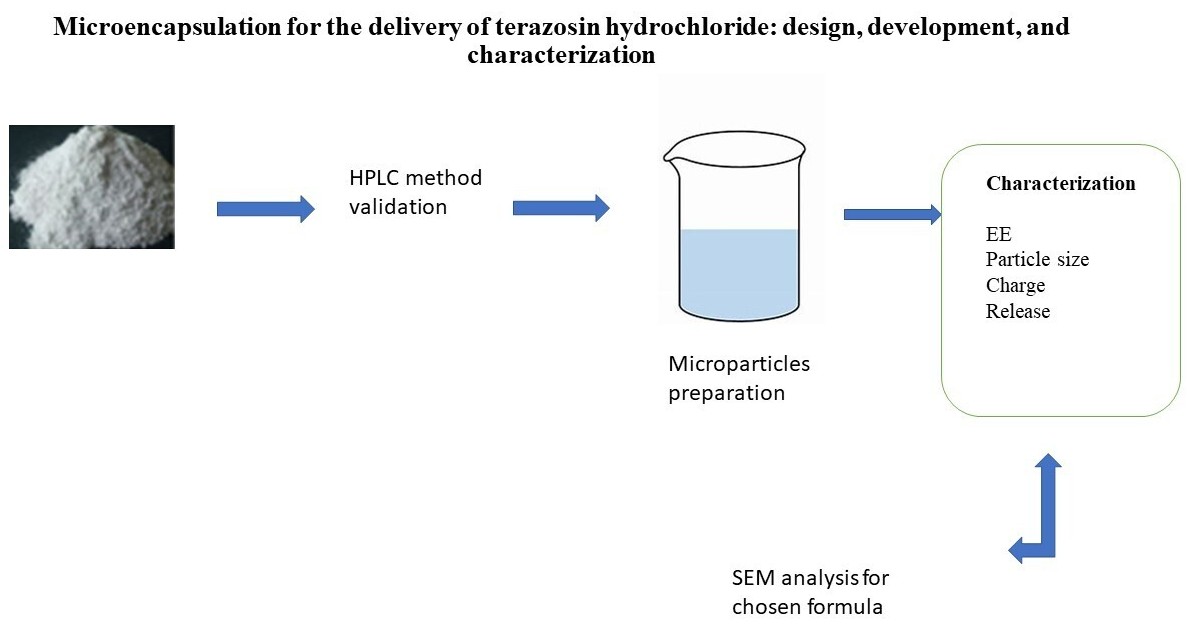
The aim of the work. Terazosin HCL, which is a selective alpha-1 antagonist, has been recommended for the treatment of benign prostatic hyperplasia-related medical conditions, including hypertension and urinary tract disorders. The purpose of this research was to create microparticles that would have a prolonged release of terazosin hydrochloride (TZ). However, TZ is a medicine that is readily soluble and has a high capability of dissolving in water.
Materials and methods. A validated HPLC method was established to assess TZ. The TZ microparticles were produced using the process of melt dispersion by utilising cetyl palmitate (CP), myristic acid (MA), Glycerol monostearate 4055 (type II) or Kolliwax® GMS II (GMS), polyethylene glycol 400 (PEG 400) as a plasticizer, and tween 80 as a stabilizing agent. Different formulations of TZ microparticles were evaluated with regard to particle size, zeta potential, and release, and morphological scanning was performed.
Results. A zeta potential that falls between -22.9 and -29.4 mV is possessed by TZ microparticles. Furthermore, the size of TZ microparticles falls between 2.11 and 5.60 µm, and the polydispersity index (PDI) was between 0.24 to 0.41. In addition, the formula (F4) that included CP, GMS, PEG 400, and Tween 80 in the proportions of 0.8:0.2:1:0.5 had the highest zeta potential (ZP) and dissolved more than 85 % of TZ after 8 hours. Therefore, F4 was chosen for the purpose of conducting morphological research.
Conclusion. Employing the use of CP, GMS, PEG 400, and Tween 80 in a ratio of 0.8:0.2:1:0.5 could result in the generation of microparticles of TZ that are the most acceptable
References
- Choudhury, N., Meghwal, M., Das, K. (2021). Microencapsulation: An overview on concepts, methods, properties and applications in foods. Food Frontiers, 2 (4), 426–442. https://doi.org/10.1002/fft2.94
- Gharsallaoui, A., Saurel, R., Chambin, O., Voilley, A. (2011). Pea (Pisum sativum, L.) Protein Isolate Stabilized Emulsions: A Novel System for Microencapsulation of Lipophilic Ingredients by Spray Drying. Food and Bioprocess Technology, 5 (6), 2211–2221. https://doi.org/10.1007/s11947-010-0497-z
- Piletti, R., Zanetti, M., Jung, G., de Mello, J. M. M., Dalcanton, F., Soares, C. et al. (2019). Microencapsulation of garlic oil by β‑cyclodextrin as a thermal protection method for antibacterial action. Materials Science and Engineering: C, 94, 139–149. https://doi.org/10.1016/j.msec.2018.09.037
- Wolska, E., Brach, M. (2022). Distribution of Drug Substances in Solid Lipid Microparticles (SLM) – Methods of Analysis and Interpretation. Pharmaceutics, 14 (2), 335. https://doi.org/10.3390/pharmaceutics14020335
- Gugu, T. H., Chime, S. A., Attama, A. A. (2015). Solid lipid microparticles: An approach for improving oral bioavailability of aspirin. Asian Journal of Pharmaceutical Sciences, 10 (5), 425–432. https://doi.org/10.1016/j.ajps.2015.06.004
- Scalia, S., Young, P. M., Traini, D. (2014). Solid lipid microparticles as an approach to drug delivery. Expert Opinion on Drug Delivery, 12 (4), 583–599. https://doi.org/10.1517/17425247.2015.980812
- HYTRIN (terazosin hydrochloride) Capsules (2005). FDA monograph. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/19057Orig1s021,%2020347Orig1s009%20lbl.pdf
- Kumar, A., Kumar, R., Mazumdar, A. (2021). Synthesis Characterization and Antibacterial Activity of Terazosin Hydrochloride Drug and Market Formulation. Research Journal of Pharmacy and Technology, 14, 4777–4782. https://doi.org/10.52711/0974-360x.2021.00831
- Yang, C. H., Raja, A. (2023). Terazosin. Treasure Island: StatPearls Publishing.
- Oestreich, M. C., Vernooij, R. W., Sathianathen, N. J., Hwang, E. C., Kuntz, G. M., Koziarz, A. et al. (2020). Alpha-blockers after shock wave lithotripsy for renal or ureteral stones in adults. Cochrane Database of Systematic Reviews, 2020 (11). https://doi.org/10.1002/14651858.cd013393.pub2
- Hundemer, G. L., Knoll, G. A., Petrcich, W., Hiremath, S., Ruzicka, M., Burns, K. D. et al. (2021). Kidney, Cardiac, and Safety Outcomes Associated With α-Blockers in Patients With CKD: A Population-Based Cohort Study. American Journal of Kidney Diseases, 77 (2), 178–189.e1. https://doi.org/10.1053/j.ajkd.2020.07.018
- Jiang, C., Jiang, X., Wang, X., Shen, J., Zhang, M., Jiang, L. et al. (2021). Transdermal iontophoresis delivery system for terazosin hydrochloride: an in vitro and in vivo study. Drug Deliv, 28 (1), 454–462. http://dx.doi.org/10.1080/10717544.2021.1889719
- Rudra, R., Ganguly, S., Dhua, M., Sikdar, B. (2013). Analytical Method Development & Validation for Estimation of Terazosin Hydrochloride Equivalent To Terazosin In Tablet Dosage Form. Pharmatutor. Available at: https://www.pharmatutor.org/articles/analytical-method-development-validation-estimation-terazosin-hydrochloride-equivalent-terazosin-in-tablet-dosage-form
- Ekambaram, P., Abdul Hasan Sathali, A. (2011). Formulation and Evaluation of Solid Lipid Nanoparticles of Ramipril. Journal of Young Pharmacists, 3 (3), 216–220. https://doi.org/10.4103/0975-1483.83765
- Eltawela, S., Abdelaziz, E., Mahmoud, M., Elghamry, H. (2021). Preparation and characterization of (−)-epigallocatechin gallate lipid based nanoparticles for enhancing availability and physical properties. Al-Azhar Journal of Pharmaceutical Sciences, 63, 17–36. http://dx.doi.org/10.21608/ajps.2021.153558
- Castro, S. R., Ribeiro, L. N. M., Breitkreitz, M. C., Guilherme, V. A., Rodrigues da Silva, G. H., Mitsutake, H. et al. (2021). A pre-formulation study of tetracaine loaded in optimized nanostructured lipid carriers. Scientific Reports, 11 (1). https://doi.org/10.1038/s41598-021-99743-6
- Rahi, F. A., Ameen, M. S. M., Jawad, K. K. M. (2022). Preparation and evaluation of lipid matrix microencapsulation for drug delivery of azilsartan kamedoxomil. ScienceRise: Pharmaceutical Science, 6 (40), 21–28. https://doi.org/10.15587/2519-4852.2022.270306
- The Pharmacopoeia of United States of America", 43th Ed., National Formulary 38, Mack publishing Co. Easton, vol. 2, Electronic version (2019).
- International Conference on Harmonization, Validation of Analytical Procedures: Text and Methodology, Q 2 (R1) (2005). ICH.
- Sangani, M. B., Patel, N. (2024). An Eco-Friendly RP-HPLC Method Development and Validation for Quantification of Favipiravir in Bulk and Tablet Dosage Form Followed by Forced Degradation Study. Journal of Chromatographic Science, 62 (5), 432–438. https://doi.org/10.1093/chromsci/bmad093
- Singh, N., Akhtar, M. J., Anchliya, A. (2021). Development and Validation of HPLC Method for Simultaneous Estimation of Reduced and Oxidized Glutathione in Bulk Pharmaceutical Formulation. Austin Journal of Analytical and Pharmaceutical Chemistry, 8 (1). https://doi.org/10.26420/austininternmed.2021.1129
- Danaei, M., Dehghankhold, M., Ataei, S., Hasanzadeh Davarani, F., Javanmard, R., Dokhani, A. et al. (2018). Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics, 10 (2), 57. https://doi.org/10.3390/pharmaceutics10020057
- Sivadasan, D., Madavan, B., Penmatsa, S. D., Bathini, S. T. (2013). Formulation and Characterization of Solid Lipid Nanoparticles of Rifampicin. Erciyes Tıp Dergisi/Erciyes Medical Journal, 35 (1), 1–5. https://doi.org/10.5152/etd.2013.01
- Kumar, M. K., Nagaraju, K., Bhanja, S., Sudhakar, M. (2014). Formulation and evaluation of Sublingual tablets of Terazosin hydrochloride. International Journal of Pharmaceutical Sciences and Research, 5 (2), 417–427. https://doi.org/10.13040/ijpsr.0975-8232.5(2).417-27
- Muskan, J., Shelendra, M., Ashok, K., Sapna, M., Anil, K. (2022). Formulation and Evaluation of Fast Dissolving Tablet of Terazosin Hydrochloride. American Journal of PharmTech Research, 12, 75–87. https://doi.org/10.5281/zenodo.7407648
- López-Iglesias, C., Quílez, C., Barros, J., Velasco, D., Alvarez-Lorenzo, C., Jorcano, J. L. et al. (2020). Lidocaine-Loaded Solid Lipid Microparticles (SLMPs) Produced from Gas-Saturated Solutions for Wound Applications. Pharmaceutics, 12 (9), 870. https://doi.org/10.3390/pharmaceutics12090870
- Ilyas, S., Maheen, S., Andleeb, M., Khan, H. U., Shah, S., Abbas, G. et al. (2023). Development, optimization and in vitro-in vivo evaluation of solid lipid microparticles for improved pharmacokinetic profile of bisoprolol. Materials Today Communications, 37, 107097. https://doi.org/10.1016/j.mtcomm.2023.107097
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Asmaa Abdelaziz Mohamed, Hiba Ezzat Hamed, Mustafa AL-Mishlab

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






