Modern approaches to typologization and modeling in the health technology assessment
DOI:
https://doi.org/10.15587/2519-4852.2024.314005Keywords:
public administration, health care system, health technology assessment, modeling, classification and typologization, typology, type (model, sample)Abstract
The aim: to develop a typology of the current management systems for health technology assessment (HTA) based on the identification of typological features in order to scientifically and practically substantiate a typological model that combines the most stable properties and can be implemented in a variety of modifications, taking into account the dynamic development of the health care system (HCS).
Materials and methods: The study used scientific publications, official information from the websites of national or regional bodies/agencies, international organizations on HTA, reports, databases and official documents of the World Health Organization (WHO). The research used the following methods: the system analysis, content analysis, institutional analysis, structural-functional analysis, generalization, comparison, systematization, classification, synthesis, typology, and modeling. To conduct a typological analysis, 34 countries were selected in which the HTA has been implemented in the decision-making process for the use and financing of medical technologies (MTs).
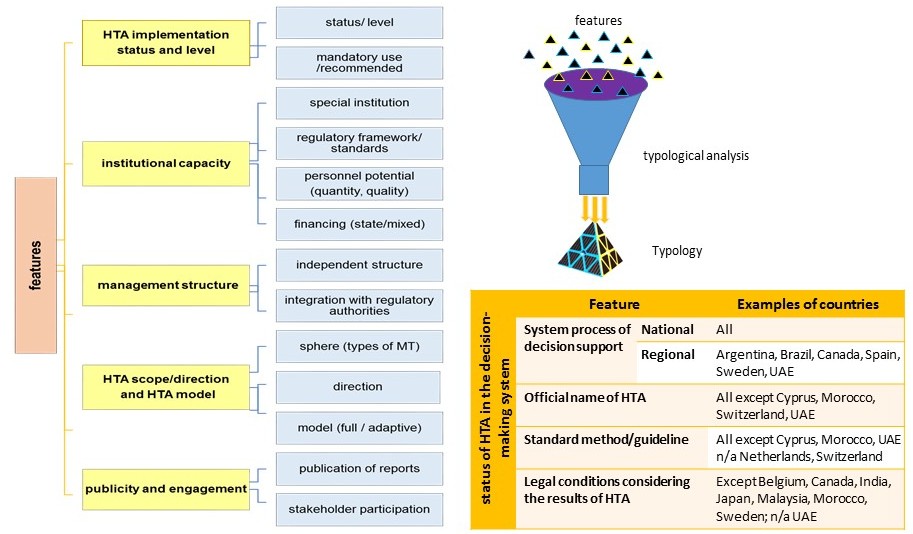
Research results. An institutional analysis of national HTA systems was conducted. The status of HTA in the national health care systems of the selected countries and, in particular, the role of HTA in the decision-making process regarding the use of certain MTs were studied. The author analyzes the institutional capacity of the HTA system (availability of a special authorized body, level of centralization/decentralization, financing, regulatory framework and human resources). The functionality and areas of activity of HTA bodies (organizations), the level of accountability, openness and interaction with various stakeholders are analyzed. The systematization and generalization of foreign experience made it possible to conduct a typological analysis by characteristic features). Four types of HTA management systems are identified (starting, centralized, decentralized, and balanced).
Conclusions: The study identifies and analyzes the areas of activity of the bodies/organizations in most countries of the world that carry out HTA in terms of their mission, vision and functionality, as well as assesses the level of their openness and interaction with various stakeholders. The scientific generalization and systematization of modern approaches and models of HTA systems made it possible to typologize them on the basis of certain characteristic classification features
References
- Tutuk, V., Nazarkina, V., Babenko, M., Nemchenko, A., Zhakipbekov, K. (2023). Assessment of medical technologies in the formation of government programs to assist patients with rare metabolic diseases. ScienceRise: Pharmaceutical Science, 5 (45), 99–108. https://doi.org/10.15587/2519-4852.2023.290218
- Fontrier, A.-M., Visintin, E., Kanavos, P. (2021). Similarities and Differences in Health Technology Assessment Systems and Implications for Coverage Decisions: Evidence from 32 Countries. PharmacoEconomics – Open, 6 (3), 315–328. https://doi.org/10.1007/s41669-021-00311-5
- Global survey on health technology assessment by national authorities (2015). WHO. Available at: https://www.who.int/publications/i/item/9789241509749
- Akehurst, R. L., Abadie, E., Renaudin, N., Sarkozy, F. (2017). Variation in Health Technology Assessment and Reimbursement Processes in Europe. Value in Health, 20 (1), 67–76. https://doi.org/10.1016/j.jval.2016.08.725
- Schuster, V. (2024). EU HTA Regulation and Joint Clinical Assessment – Threat or Opportunity? Journal of Market Access & Health Policy, 12 (2), 100–104. https://doi.org/10.3390/jmahp12020008
- Health Technology Assessment and Health Benefit Package Survey 2020/2021. Available at: https://www.who.int/teams/health-systems-governance-and-financing/economic-analysis/health-technology-assessment-and-benefit-package-design/survey-homepage
- Heryliv, D. Yu. (2013). Differentiation of typology and classification of state: problematic issues. Naukovyi visnyk Uzhhorodskoho natsionalnoho universytetu. Seriia Pravo, 22 (1), 19‒23.
- Nastasyak, I. (2015). Relationship of concept «typologization» with related concepts. Visnyk of the Lviv University. Series Law, 61, 37‒44. Available at: http://nbuv.gov.ua/UJRN/Vlnu_yu_2015_61_8
- Reibling, N., Ariaans, M., Wendt, C. (2019). Worlds of Healthcare: A Healthcare System Typology of OECD Countries. Health Policy, 123 (7), 611–620. https://doi.org/10.1016/j.healthpol.2019.05.001
- Wendt, C.; Levy, A., Goring, S., Gatsonis, C., Sobolev, B., van Ginneke,n E., Busse, R. (Eds.) (2019). Health System Typologies. Health Services Research. New York: Springer, 927–937. https://doi.org/10.1007/978-1-4939-8715-3_21
- Wendt, C.; van Ginneken, E., Busse, R. (Eds.) (2018). Health System Typologies. Health Care Systems and Policies. Health Services Research. New York: Springer. https://doi.org/10.1007/978-1-4614-6419-8_21-1
- Burau, V., Blank, R. H., Pavolini, E.; Kuhlmann, E., Blank, R. H., Bourgeault, I. L., Wendt, C. (Eds.) (2015). Typologies of Healthcare Systems and Policies. The Palgrave International Handbook of Healthcare Policy and Governance. London: Palgrave Macmillan, 101–115. https://doi.org/10.1057/9781137384935_7
- de Carvalho, G., Schmid, A., Fischer, J. (2020). Classifications of health care systems: Do existing typologies reflect the particularities of the Global South? Global Social Policy, 21 (2), 278–300. https://doi.org/10.1177/1468018120969315
- Khomenko, V. M., Nemchenko, A. S., Kosiachenko, K. L. (2007). Typolohiia derzhavno-upravlinskykh vidnosyn u farmatsii: pytannia teorii ta praktyka. Farmatsevtychnyi zhurnal, 4, 3‒9.
- Evans, T. G., Ahmed, S. M.; Raviglione, M. C. B., Tediosi, F., Villa, S., Casamitjana, N., Plasència, A. (Eds.) (2023). Governance of Health Systems. Global Health Essentials. Sustainable Development Goals Series. Springer: Cham, 285–289. https://doi.org/10.1007/978-3-031-33851-9_43
- Angelis, A., Lange, A., Kanavos, P. (2017). Using health technology assessment to assess the value of new medicines: results of a systematic review and expert consultation across eight European countries. The European Journal of Health Economics, 19 (1), 123–152. https://doi.org/10.1007/s10198-017-0871-0
- Chamova, J. (2018). Mapping of HTA national organisations, programmes & processes in EU & Norway. EC, Directorate. General for Health & Food Safety, Publ. Office. https://doi.org/10.2875/5065
- Kristensen, F. B. (2017). Mapping of HTA methodologies in EU and Norway. EC, Directorate-General for Health and Food Safety, Publ. Office. https://doi.org/10.2875/472312
- Health Technology Assessment (HTA) in the Nordic countries. Introduction to and Status of HTA’s Role in the Value Chain of Medical Technology (2017). Nordic Medtech Growth, 48.
- Nazarkina, V. M., Nemchenko, A. S., Kosiachenko, K. L., Babenko, M. M.; Nemchenko, A. S. (Ed.) (2022). Metodolohiia tsinoutvorennia na likarski zasoby v systemi okhorony zdorovia. Kyiv: Farmatsevt Praktyk, 288.
- Nemzoff, C., Shah, H. A., Heupink, L. F., Regan, L., Ghosh, S., Pincombe, M. et al. (2023). Adaptive Health Technology Assessment: A Scoping Review of Methods. Value in Health, 26 (10), 1549–1557. https://doi.org/10.1016/j.jval.2023.05.017
- Kristensen, F. B. (2012). Development of European HTA: from vision to EUnetHTA. Michael, 9, 147–156. Available at: http://www.dnms.no/index.php?seks_id=149347&a=1
- Henshall, C. (2012). Describe decision-making systems, assess health technology assessment reports. International Journal of Technology Assessment in Health Care, 28 (2), 168. https://doi.org/10.1017/s0266462312000177
- Allen, N., Pichler, F., Wang, T., Patel, S., Salek, S. (2013). Development of archetypes for non-ranking classification and comparison of European National Health Technology Assessment systems. Health Policy, 113 (3), 305–312. https://doi.org/10.1016/j.healthpol.2013.09.007
- WHA67.23 – Health Intervention and Technology Assessment in Support of Universal Health Coverage. WHA Resolution; Sixty-seventh World Health Assembly (2014). World Health Organization. Available at: http://apps.who.int/medicinedocs/documents/s21463en/s21463en.pdf
- Allen, N., Liberti, L., Walker, S. R., Salek, S. (2017). A Comparison of Reimbursement Recommendations by European HTA Agencies: Is There Opportunity for Further Alignment? Frontiers in Pharmacology, 8. https://doi.org/10.3389/fphar.2017.00384
- Kristensen, F. B., Nielsen, C. P., Panteli, D.; Busse, R., Klazinga, N., Panteli, D. et al. (Ed.) (2019). Regulating the input – Health Technology Assessment. Improving healthcare quality in Europe: Characteristics, effectiveness and implementation of different strategies. Copenhagen: European Observatory on Health Systems and Policies. (Health Policy Series No. 53). Available at: https://www.ncbi.nlm.nih.gov/books/NBK549272/
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Mykhailo Babenko, Kostyantyn Kosyachenko

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






