An approach to the technological process validation of manufacturing medical devices using the example of injectable implants based on hyaluronic acid
DOI:
https://doi.org/10.15587/2519-4852.2024.319456Keywords:
technological process, validation, diagram Ishikawa, medical devices, injectable implants, hyaluronic acidAbstract
The aim. Technological process validation of manufacturing medical devices is a necessary condition for confirming the ability to continuously produce high-quality medical devices, reduce or eliminate the number of defects, improve the level of product quality, and is also one of the main requirements for product certification on the European Union market. Given the wide variety of medical device types (from patches to pre-filled syringes), unlike medicinal products, the validation procedure for medical devices does not have clear recommendations and guidelines.
Materials and methods. The subject of this article is the determination of the approach to the technological process validation of manufacturing medical devices using the example of injectable implants based on cross-linking hyaluronic acid, based on the experience of batch production of the specified type of products on an industrial scale and the regulatory requirements of Ukraine and the European Union.
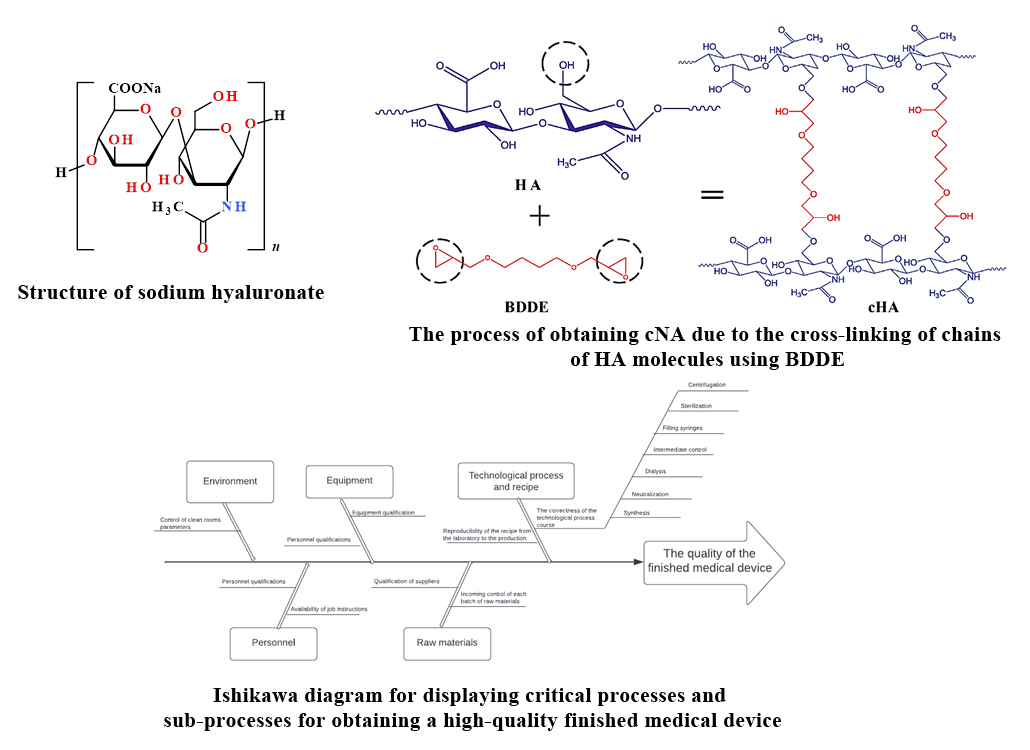
Results. The article presents information about the nature of hyaluronic acid, its structure, sources and methods of production, and the scope of application.
Determination of critical points of the technological process was carried out by the method of risk assessment using the approach of forming the Ishikawa diagram, i.e. "analysis of cause-and-effect relationships ".
The main stages of the analysis of causal relationships are the following:
– determination of the process that is subject to analysis (obtaining high-quality finished products) and sub-processes that have an impact on the final result;
– determination of the main categories of impact on the process, displayed by blocks on the Ishikawa diagram.
The result of such an analysis is displayed in the form of the above-mentioned diagram Ishikawa ("fishbone").
Sub-processes that have the main influence on it were determined. These elements are the critical points that will be subject to validation. The impact of each of these elements, their key parameters and permissible operating ranges are described in the article.
Conclusions. The sub-processes that have the main impact on the technological process of manufacturing the medical device are identified. These elements are critical points that are subject to validation. The article describes the impact of each of these elements, their main parameters and permissible operating ranges, and also presents the validation process and confirmation of the validity of the corresponding technology
References
- ISO 14971:2019 Medical devices – Application of risk management to medical devices (2019). Available at: https://www.iso.org/standard/72704.html
- European Pharmacopoeia 11.0. Available at: https://www.edqm.eu/en/european-pharmacopoeia-ph.-eur.-11th-edition
- Romagnoli, M., Belmontesi, M. (2008). Hyaluronic acid–based fillers: theory and practice. Clinics in Dermatology, 26 (2), 123–159. https://doi.org/10.1016/j.clindermatol.2007.09.001
- Zhang, Y., Tan, W., Zhang, Y., Mao, H., Shi, S., Duan, L., Wang, H., Yu, J. (2019). Ultrasensitive and selective detection of Staphylococcus aureus using a novel IgY-based colorimetric platform. Biosensors and Bioelectronics, 142, 111570. https://doi.org/10.1016/j.bios.2019.111570
- Patil, K. P., Kamalja, K. K., Chaudhari, B. L. (2011). Optimization of medium components for hyaluronic acid production by Streptococcus zooepidemicus MTCC 3523 using a statistical approach. Carbohydrate Polymers, 86 (4), 1573–1577. https://doi.org/10.1016/j.carbpol.2011.06.065
- Huang, W.-C., Chen, S.-J., Chen, T.-L. (2008). Production of hyaluronic acid by repeated batch fermentation. Biochemical Engineering Journal, 40 (3), 460–464. https://doi.org/10.1016/j.bej.2008.01.021
- Ogrodowski, C. S., Hokka, C. O., Santana, M. H. A. (2005). Production of hyaluronic acid. Applied Biochemistry and Biotechnology, 122, 753–761.
- Liu, L., Du, G., Chen, J., Zhu, Y., Wang, M., Sun, J. (2009). Microbial production of low molecular weight hyaluronic acid by adding hydrogen peroxide and ascorbate in batch culture of Streptococcus zooepidemicus. Bioresource Technology, 100 (1), 362–367. https://doi.org/10.1016/j.biortech.2008.05.040
- Rangaswamy, V., Jain, D. (2007). An efficient process for production and purification of hyaluronic acid from Streptococcus equi subsp. zooepidemicus. Biotechnology Letters, 30 (3), 493–496. https://doi.org/10.1007/s10529-007-9562-8
- Liu, W., Ma, M., Lei, Z., Xiong, Z., Tao, T., Lei, P. et al. (2022). Intra-articular injectable hydroxypropyl chitin/hyaluronic acid hydrogel as bio-lubricant to attenuate osteoarthritis progression. Materials & Design, 217, 110579. https://doi.org/10.1016/j.matdes.2022.110579
- Belk, J. W., Lim, J. J., Keeter, C., McCulloch, P. C., Houck, D. A., McCarty, E. C. et al. (2023). Patients With Knee Osteoarthritis Who Receive Platelet-Rich Plasma or Bone Marrow Aspirate Concentrate Injections Have Better Outcomes Than Patients Who Receive Hyaluronic Acid: Systematic Review and Meta-analysis. Arthroscopy: The Journal of Arthroscopic & Related Surgery, 39 (7), 1714–1734. https://doi.org/10.1016/j.arthro.2023.03.001
- Gobbi, A., Morales, M., Avio, G., D’Ambrosi, R. (2022). Double-blinded prospective randomized clinical trial in knee joint osteoarthritis treatment: safety assessment and performance of trehalose hyaluronic acid versus standard infiltrative therapy based on medium-weight sodium hyaluronate. Journal of Cartilage & Joint Preservation, 2 (3), 100043. https://doi.org/10.1016/j.jcjp.2022.100043
- Rai, V. K., Saha, I., Alam, M., Nishchaya, K., Ghosh, G., Rath, G. (2023). Microneedle arrays for cutaneous and transcutaneous drug delivery, disease diagnosis, and cosmetic aid. Journal of Drug Delivery Science and Technology, 79, 104058. https://doi.org/10.1016/j.jddst.2022.104058
- Shimizu, Y., Ntege, E. H., Sunami, H. (2022). Current regenerative medicine-based approaches for skin regeneration: A review of literature and a report on clinical applications in Japan. Regenerative Therapy, 21, 73–80. https://doi.org/10.1016/j.reth.2022.05.008
- Huynh, A., Priefer, R. (2020). Hyaluronic acid applications in ophthalmology, rheumatology, and dermatology. Carbohydrate Research, 489, 107950. https://doi.org/10.1016/j.carres.2020.107950
- Soares, D. J., Zuliani, G. F. (2022). Orbital post-septal hyaluronic acid: An iatrogenic etiology compounding lower eyelid steatoblepharon. JPRAS Open, 34, 173–177. https://doi.org/10.1016/j.jpra.2022.09.010
- Mateo-Orobia, A. J., del Prado Sanz, E., Blasco-Martínez, A., Pablo-Júlvez, L. E., Farrant, S., Chiambaretta, F. (2023). Efficacy of artificial tears containing trehalose and hyaluronic acid for dry eye disease in women aged 42–54 versus ≥ 55 years. Contact Lens and Anterior Eye, 46 (4), 101845. https://doi.org/10.1016/j.clae.2023.101845
- De Boulle, K., Glogau, R., Kono, T., Nathan, M., Tezel, A., Roca-Martinez, J.-X. et al. (2013). A Review of the Metabolism of 1,4-Butanediol Diglycidyl Ether-Crosslinked Hyaluronic Acid Dermal Fillers. Dermatologic Surgery, 39 (12), 1758–1766. https://doi.org/10.1111/dsu.12301
- Schuurmans, Carl. C. L., Mihajlovic, M., Hiemstra, C., Ito, K., Hennink, W. E., Vermonden, T. (2021). Hyaluronic acid and chondroitin sulfate (meth)acrylate-based hydrogels for tissue engineering: Synthesis, characteristics and pre-clinical evaluation. Biomaterials, 268, 120602. https://doi.org/10.1016/j.biomaterials.2020.120602
- Schanté, C. E., Zuber, G., Herlin, C., Vandamme, T. F. (2011). Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydrate Polymers, 85 (3), 469–489. https://doi.org/10.1016/j.carbpol.2011.03.019
- Zhang, J. N., Chen, B. Z., Ashfaq, M., Zhang, X. P., Guo, X. D. (2018). Development of a BDDE-crosslinked hyaluronic acid based microneedles patch as a dermal filler for anti-ageing treatment. Journal of Industrial and Engineering Chemistry, 65, 363–369. https://doi.org/10.1016/j.jiec.2018.05.007
- Al-Sibani, M., Al-Harrasi, A., Neubert, R. H. H. (2016). Study of the effect of mixing approach on cross-linking efficiency of hyaluronic acid-based hydrogel cross-linked with 1,4-butanediol diglycidyl ether. European Journal of Pharmaceutical Sciences, 91, 131–137. https://doi.org/10.1016/j.ejps.2016.06.010
- Kenne, L., Gohil, S., Nilsson, E. M., Karlsson, A., Ericsson, D., Helander Kenne, A., Nord, L. I. (2013). Modification and cross-linking parameters in hyaluronic acid hydrogels – Definitions and analytical methods. Carbohydrate Polymers, 91 (1), 410–418. https://doi.org/10.1016/j.carbpol.2012.08.066
- ISO 13485:2016 Medical devices – Quality management systems – Requirements for regulatory purposes (2016). Available at: https://www.iso.org/ru/standard/59752.html
- Zhao, Y., Sheng, K., Wang, Z., Zhang, X., HengyiYang, Miao, R. (2017). Process Validation and Revalidation in Medical Device Production. Procedia Engineering, 174, 686–692. https://doi.org/10.1016/j.proeng.2017.01.207
- Pitarresi, G., Palumbo, F. S., Tripodo, G., Cavallaro, G., Giammona, G. (2007). Preparation and characterization of new hydrogels based on hyaluronic acid and α,β-polyaspartylhydrazide. European Polymer Journal, 43 (9), 3953–3962. https://doi.org/10.1016/j.eurpolymj.2007.06.027
- DSTU IEC/ISO 31010:2013 Risk management. Methods of general risk assessment. Available at: https://khoda.gov.ua/image/catalog/files/dstu%2031010.pdf
- ISO 14644-1:2015 Cleanrooms and associated controlled environments. Part 1: Classification of air cleaness. Available at: https://www.iso.org/standard/53394.html
- On the approval of the Technical Regulation on medical devices (2013). Resolution No. 753. 02.10.2013.
- Medical Device Regulation 2017/745. Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32017R0745
- U.S. Food and Drug Adminictration. Available at: https://www.fda.gov/
- GHTF/SG3/N99-10:2015 Quality management systems. Guidance on process validation. The document is harmonized to GHTF/SG3/N99-10:2004 Quality Management Systems – Process Validation Guidance (2015). Edition 2.
- EMA/CHMP/CVMP/QWP/749073/2016 EMA Guideline on process validation for finished products – information and data to be provided in regulatory submission (2016).
- ICH Q7 Current Step 4 version (2000).
- EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use Volume 4 (2022). Available at: https://health.ec.europa.eu/latest-updates/eudralex-volume-4-eu-guidelines-good-manufacturing-practice-medicinal-products-human-and-veterinary-2022-02-21_en
- СТ-Н МОЗУ 42-4.0:2015 MEDICINES Good Manufacturing Practice.
- Inna Bondarets, Lyudmila Sidorenko, Victoriya Georgiyants, Volodymyr Mishchenko. Bondarets, I., Sidorenko, L. et al. (2022). Regulatory and risk oriented approach to the design and development of medical devices in accordance with Ukraine regulations. Pharmacia, 69 (2), 493–500. https://doi.org/10.3897/pharmacia.69.e82316
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Inna Bondarets, Lyudmila Sidorenko, Olga Antonenko, Serhii Lebed, Victoriya Georgiyants

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






