Impact of the natural oligoribonucleotides in complexes with D-mannitol on tumor growth and expression of immune cell markers in a mouse melanoma В16 model
DOI:
https://doi.org/10.15587/2519-4852.2025.326767Keywords:
natural oligoribonucleotides, B16 melanoma, tumour formation, cytotoxicity, in vivo model, cancerAbstract
Cancer remains a leading cause of mortality worldwide, with chronic inflammation playing a critical role in tumour initiation and progression. Natural oligoribonucleotides in complexes with D-mannitol (ORN-D-M), derived from yeast RNA, have demonstrated anti-inflammatory and immunomodulatory effects in various disease models. Previous studies have shown their cytotoxicity against the B16 mouse melanoma cell line in vitro. This study aimed to evaluate the antitumor properties of ORN-D-M in a B16 mouse melanoma model.
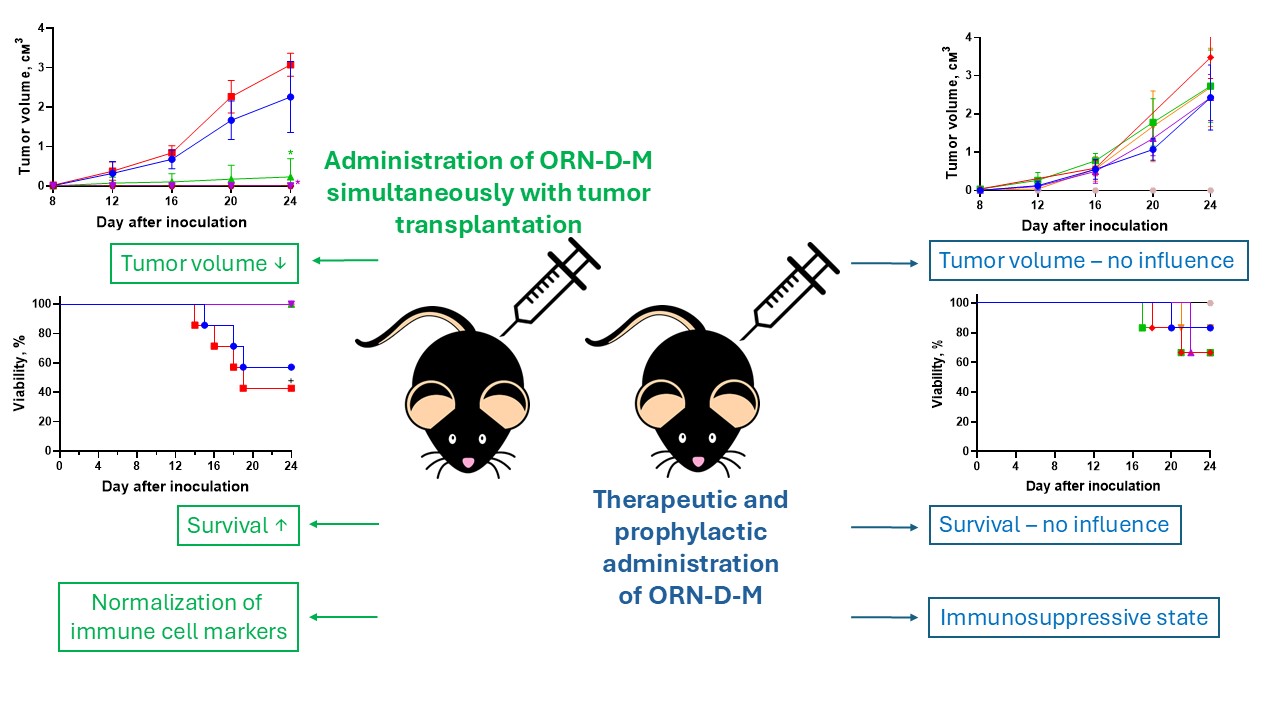
Materials and methods. The B16 melanoma model was established by transplantation of B16 cells into C57BL/6 mice. ORN-D-M was administered at varying doses via different routes (subcutaneous, oral, intraperitoneal), under prophylactic and therapeutic regimens, and simultaneously with transplantation of malignant cells. Tumour growth dynamics, survival, and gene expression of immune cell markers in peripheral blood (Cd3, Cd247, Cd4, Cd8, Cd68) using RT-qPCR were assessed.
Results. It was shown that simultaneous administration of ORN-D-M with tumour cell transplantation dose-dependently inhibited tumour formation and improved survival. However, neither therapeutic nor prophylactic administration after tumour transplantation showed significant effects. Additionally, ORN-D-M did not affect the mRNA expression of immune cell markers during the late stage of B16 melanoma.
Conclusion. ORN-D-M exhibits a dose-dependent cytotoxic effect when administered simultaneously with tumour cells but lacks efficacy in therapeutic or prophylactic regimens. Future studies should focus on optimizing targeted delivery systems to enhance drug stability and effectiveness in cancer therapy
Supporting Agency
- This work was supported by Simons Support Grant (1290589, Ivanna Prylutska, Zenoviy Tkachuk).
References
- Aggarwal, B. B., Vijayalekshmi, R. V., Sung, B. (2009). Targeting Inflammatory Pathways for Prevention and Therapy of Cancer: Short-Term Friend, Long-Term Foe. Clinical Cancer Research, 15 (2), 425–430. https://doi.org/10.1158/1078-0432.ccr-08-0149
- Grivennikov, S. I., Greten, F. R., Karin, M. (2010). Immunity, Inflammation, and Cancer. Cell, 140 (6), 883–899. https://doi.org/10.1016/j.cell.2010.01.025
- Gerashchenko, G. V., Kashuba, V. I., Tukalo, M. A. (2023). Key models and theories of carcinogenesis. Biopolymers and Cell, 39 (3), 161–169. https://doi.org/10.7124/bc.000a99
- Fitzgerald, K. A., Kagan, J. C. (2020). Toll-like Receptors and the Control of Immunity. Cell, 180 (6), 1044–1066. https://doi.org/10.1016/j.cell.2020.02.041
- Urban-Wojciuk, Z., Khan, M. M., Oyler, B. L., Fåhraeus, R., Marek-Trzonkowska, N., Nita-Lazar, A. et al. (2019). The Role of TLRs in Anti-cancer Immunity and Tumor Rejection. Frontiers in Immunology, 10. https://doi.org/10.3389/fimmu.2019.02388
- Rolfo, C., Giovannetti, E., Martinez, P., McCue, S., Naing, A. (2023). Applications and clinical trial landscape using Toll-like receptor agonists to reduce the toll of cancer. Npj Precision Oncology, 7 (1). https://doi.org/10.1038/s41698-023-00364-1
- Kawai, K., Miyazaki, J., Joraku, A., Nishiyama, H., Akaza, H. (2013). Bacillus Calmette-Guerin (BCG) Immunotherapy for Bladder Cancer: Current Understanding and Perspectives on Engineered BCG Vaccine. Cancer Science, 104(1), 22–27. Portico. https://doi.org/10.1111/cas.12075
- Salaun, B., Coste, I., Rissoan, M.-C., Lebecque, S. J., Renno, T. (2006). TLR3 Can Directly Trigger Apoptosis in Human Cancer Cells. The Journal of Immunology, 176 (8), 4894–4901. https://doi.org/10.4049/jimmunol.176.8.4894
- Hou, J., Karin, M., Sun, B. (2021). Targeting cancer-promoting inflammation – have anti-inflammatory therapies come of age? Nature Reviews Clinical Oncology, 18 (5), 261–279. https://doi.org/10.1038/s41571-020-00459-9
- Thiruchenthooran, V., Sánchez-López, E., Gliszczyńska, A. (2023). Perspectives of the Application of Non-Steroidal Anti-Inflammatory Drugs in Cancer Therapy: Attempts to Overcome Their Unfavorable Side Effects. Cancers, 15 (2), 475. https://doi.org/10.3390/cancers15020475
- Tkachuk, Z. (2013). Pat. No. US8420617B2 USA. Multiantivirus Compound, Composition and Method for Treatment of Virus Diseases. published: 16.04.2013.
- Tkachuk, Z. Yu., Tkachuk, V. V., Tkachuk, L. V. (2006). The study on membrane-stabilizing and anti-inflammatory actions of yeast RNA in vivo and in vitro. Biopolymers and Cell, 22 (2), 109–116. https://doi.org/10.7124/bc.000723
- Frolov, V. M., Sotska, Ya. A., Kruglova, O. V., Tkachuk, Z. Yu. (2012). Influence of antiviral drug nuclex on the cellular immunity at the patients with chronic viral hepatitis C. Ukrainskyi morfolohichnyi almanakh, 10, 99–105.
- Kraievska, I. M., Tkachuk, Z. Yu. (2023). Effect of complexes of natural oligoribonucleotides with D-mannitol on the viability of cell cultures of different origin. Biopolymers and Cell, 39 (3), 220–230. https://doi.org/10.7124/bc.000a93
- Prylutska, I. M., Tkachuk, Z. Yu. (2024). Oligoribonucleotides in complexes with D-mannitol alter cell cycle and cause apoptosis in murine melanoma B16 cells. Biopolymers and Cell, 40 (2), 118–126. https://doi.org/10.7124/bc.000ab2
- Overwijk, W. W., Restifo, N. P. (2000). B16 as a Mouse Model for Human Melanoma. Current Protocols in Immunology, 39 (1). https://doi.org/10.1002/0471142735.im2001s39
- Stefanov, O. V.; Litvinova, N. V., Filonenko-Patrusheva, M. A., Frantsuzova, S. B., Khrapak, V. V. (Eds.) (2001). Doklinichni Doslidzhennnia Likarskykh Zasobiv. Kyiv: Vydavnychyi dim «Avitsena».
- Workman, P., Aboagye, E. O., Balkwill, F., Balmain, A., Bruder, G., Chaplin, D. J. et al. (2010). Guidelines for the welfare and use of animals in cancer research. British Journal of Cancer, 102 (11), 1555–1577. https://doi.org/10.1038/sj.bjc.6605642
- Faustino-Rocha, A., Oliveira, P. A., Pinho-Oliveira, J., Teixeira-Guedes, C., Soares-Maia, R., da Costa, R. G. et al. (2013). Estimation of rat mammary tumor volume using caliper and ultrasonography measurements. Lab Animal, 42 (6), 217–224. https://doi.org/10.1038/laban.254
- Kulyk, O., Krivoshey, A., Kolosova, O., Prylutska, I., Vasiliu, T., Puf, R. et al. (2024). Nucleic acid-binding bis-acridine orange dyes with improved properties for bioimaging and PCR applications. Journal of Materials Chemistry B, 12 (46), 11968–11982. https://doi.org/10.1039/d4tb01775g
- Gerashchenko, G. V., Vagina, I. M., Vagin, Yu. V., Kashuba, V. I. (2020). Pattern of expression of immune- and stroma-associated genes in blood of mice with experimental B16 melanoma. The Ukrainian Biochemical Journal, 92 (1), 5–11. https://doi.org/10.15407/ubj92.01.005
- Kamran, N., Li, Y., Sierra, M., Alghamri, M. S., Kadiyala, P., Appelman, H. D., Edwards, M., Lowenstein, P. R., Castro, M. G. (2017). Melanoma induced immunosuppression is mediated by hematopoietic dysregulation. OncoImmunology, 7 (3), e1408750. https://doi.org/10.1080/2162402x.2017.1408750
- King, K. Y., Goodell, M. A. (2011). Inflammatory modulation of HSCs: viewing the HSC as a foundation for the immune response. Nature Reviews Immunology, 11 (10), 685–692. https://doi.org/10.1038/nri3062
- Duan, T., Du, Y., Xing, C., Wang, H. Y., Wang, R.-F. (2022). Toll-Like Receptor Signaling and Its Role in Cell-Mediated Immunity. Frontiers in Immunology, 13. https://doi.org/10.3389/fimmu.2022.812774
- Takizawa, H., Boettcher, S., Manz, M. G. (2012). Demand-adapted regulation of early hematopoiesis in infection and inflammation. Blood, 119 (13), 2991–3002. https://doi.org/10.1182/blood-2011-12-380113
- Nishii, N., Tachinami, H., Kondo, Y., Xia, Y., Kashima, Y., Ohno, T. et al. (2018). Systemic administration of a TLR7 agonist attenuates regulatory T cells by dendritic cell modification and overcomes resistance to PD-L1 blockade therapy. Oncotarget, 9 (17), 13301–13312. https://doi.org/10.18632/oncotarget.24327
- Lemke-Miltner, C. D., Blackwell, S. E., Yin, C., Krug, A. E., Morris, A. J., Krieg, A. M., Weiner, G. J. (2020). Antibody Opsonization of a TLR9 Agonist–Containing Virus-like Particle Enhances in Situ Immunization. The Journal of Immunology, 204 (5), 1386–1394. https://doi.org/10.4049/jimmunol.1900742
- Arca, M. J., Krauss, J. C., Strome, S. E., Cameron, M. J., Chang, A. E. (1996). Diverse manifestations of tumorigenicity and immunogenicity displayed by the poorly immunogenic B16-BL6 melanoma transduced with cytokine genes. Cancer Immunology, Immunotherapy, 42 (4), 237–245. https://doi.org/10.1007/s002620050276
- Medler, T. R., Blair, T. C., Crittenden, M. R., Gough, M. J. (2021). Defining Immunogenic and Radioimmunogenic Tumors. Frontiers in Oncology, 11. https://doi.org/10.3389/fonc.2021.667075
- Voskoglou-Nomikos, T., Pater, J., Seymour, L. (2003). Clinical Predictive Value of the in Vitro Cell Line, Human Xenograft, and Mouse Allograft Preclinical Cancer Models. Clinical Cancer Research, 9, 4227–4239.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Ivanna Prylutska, Zenoviy Tkachuk

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






