Combined methanolic extracts of selected medicinal plants in southern Philippines as a potential therapeutic tool for diabetes and obesity
DOI:
https://doi.org/10.15587/2519-4852.2025.328418Keywords:
Type 2 Diabetes, oxidative stress, obesity, AGEs, metabolic diseaseAbstract
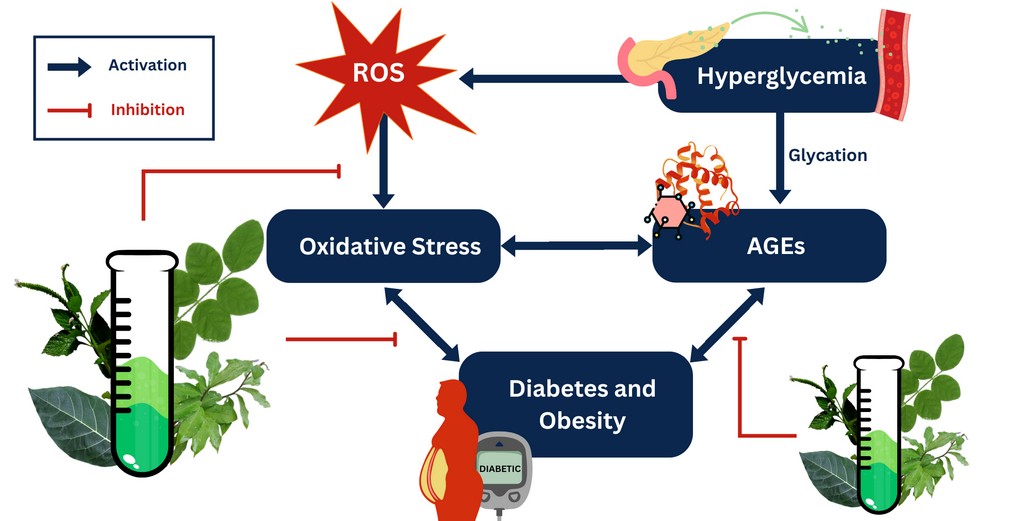
Type 2 Diabetes (T2D) is a significant public health problem with an increasing number of cases over the years. With this, the search for cheaper and natural alternatives also continues. Oxidative stress, obesity, and advanced glycation end products (AGEs) formation contribute to T2D pathogenesis.
The aim. This study employs a range of functional assays to assess the antidiabetic, antioxidant, anti-obesity, and antiglycation activities of air-dried leaf methanolic extracts from the combined extract (CoM) of Clitoria ternatea, Ficus septica, Heliotropium indicum, and Celosia ignea. Additionally, quantitative screening was conducted to determine the presence of key bioactive secondary metabolites, particularly flavonoids and phenolic compounds.
Materials and methods. Glucose adsorption and glucose diffusion were utilized to measure the antihyperglycemic effects; BSA protein-methylglyoxal and BSA-glucose reactions were used as models for the glycation studies; the pancreatic lipase enzyme inhibition was employed to assess the sample extracts’ potential lipid-lowering effects; and quantitative phytochemical screening for total phenolic compounds and total flavonoid contents was conducted for initial characterization of phytoconstituents presents.
Results. This study reported the glucose adsorption capacities of CoM at various concentrations (25, 50, 100 ppm) indicative of its potential antihyperglycemic effects. An in vitro glucose diffusion assay, on the other hand, showed a negative result (1.82±0.06 at 100-ppm) relative to the control. The CoM also exhibited antioxidant capacities via iron-reducing assay and H2O2 scavenging activity (57.86±8.28 % at 25-ppm). PPL inhibition was evaluated to indicate potential antiobesity and this study reported that CoM (75-ppm) inhibited 52.13±7.16 % enzyme activity. Antiglycation tests revealed that CoM extracts are potential inhibitors of AGEs formation as it (100-ppm) inhibited 72.23±2.71 % of the glycation (BSA-glucose model) and 55.46±13.43 % (BSA-MGO model). Phytochemical screening results support the presented properties with TPC and TFC of 11.29±2.10 GAE mg/ g sample and 5.83±0.03 QE mg/ g sample, respectively.
Conclusion. Overall, the combined methanolic plant extracts, CoM, may be utilized as a treatment strategy for oxidative stress-driven metabolic disorders such as diabetes and obesity. While this provides promising results, further investigation must still be done on the bioactive compounds of the sample
References
- DeFronzo, R. A. (2009). From the Triumvirate to the Ominous Octet: A New Paradigm for the Treatment of Type 2 Diabetes Mellitus. Diabetes, 58 (4), 773–795. https://doi.org/10.2337/db09-9028
- Cho, N. H., Shaw, J. E., Karuranga, S., Huang, Y., da Rocha Fernandes, J. D., Ohlrogge, A. W., Malanda, B. (2018). IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Research and Clinical Practice, 138, 271–281. https://doi.org/10.1016/j.diabres.2018.02.023
- Kamalakkannan, N., Prince, P. S. M. (2006). Rutin improves the antioxidant status in streptozotocin-induced diabetic rat tissues. Molecular and Cellular Biochemistry, 293 (1-2), 211–219. https://doi.org/10.1007/s11010-006-9244-1
- Kawser Hossain, M., Abdal Dayem, A., Han, J., Yin, Y., Kim, K., Kumar Saha, S. et al. (2016). Molecular Mechanisms of the Anti-Obesity and Anti-Diabetic Properties of Flavonoids. International Journal of Molecular Sciences, 17 (4), 569. https://doi.org/10.3390/ijms17040569
- International Diabetes Federation. IDF Diabetes Atlas. Available at: https://diabetesatlas.org/idfawp/resource-files/2021/07/IDF_Atlas_10th_Edition_2021.pdf Lat accessed: 04.05.2022
- Saxena, A., Vikram, N. K. (2004). Role of Selected Indian Plants in Management of Type 2 Diabetes: A Review. The Journal of Alternative and Complementary Medicine, 10 (2), 369–378. https://doi.org/10.1089/107555304323062365
- Efferth, T. (2007). Willmar Schwabe Award 2006: Antiplasmodial and Antitumor Activity of Artemisinin – From Bench to Bedside. Planta Medica, 73 (4), 299–309. https://doi.org/10.1055/s-2007-967138
- Ou, S., Kwok, K., Li, Y., Fu, L. (2001). In Vitro Study of Possible Role of Dietary Fiber in Lowering Postprandial Serum Glucose. Journal of Agricultural and Food Chemistry, 49 (2), 1026–1029. https://doi.org/10.1021/jf000574n
- Adiotomre, J., Eastwood, M., Edwards, C., Brydon, W. (1990). Dietary fiber: in vitro methods that anticipate nutrition and metabolic activity in humans. The American Journal of Clinical Nutrition, 52 (1), 128–134. https://doi.org/10.1093/ajcn/52.1.128
- Oyaizu, M. (1986). Studies on products of browning reaction. Antioxidative activities of products of browning reaction prepared from glucosamine. The Japanese Journal of Nutrition and Dietetics, 44 (6), 307–315. https://doi.org/10.5264/eiyogakuzashi.44.307
- Ruch, R. J., Cheng, S., Klaunig, J. E. (1989). Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis, 10 (6), 1003–1008. https://doi.org/10.1093/carcin/10.6.1003
- Kim, Y. S., Lee, Y. M., Kim, H., Kim, J., Jang, D. S., Kim, J. H., Kim, J. S. (2010). Anti-obesity effect of Morus bombycis root extract: Anti-lipase activity and lipolytic effect. Journal of Ethnopharmacology, 130 (3), 621–624. https://doi.org/10.1016/j.jep.2010.05.053
- Zheng, C.-D., Duan, Y.-Q., Gao, J.-M., Ruan, Z.-G. (2010). Screening for Anti-lipase Properties of 37 Traditional Chinese Medicinal Herbs. Journal of the Chinese Medical Association, 73 (6), 319–324. https://doi.org/10.1016/s1726-4901(10)70068-x
- Miroliaei, M., Khazaei, S., Moshkelgosha, S., Shirvani, M. (2011). Inhibitory effects of Lemon balm (Melissa officinalis, L.) extract on the formation of advanced glycation end products. Food Chemistry, 129 (2), 267–271. https://doi.org/10.1016/j.foodchem.2011.04.039
- Ni, M., Song, X., Pan, J., Gong, D., Zhang, G. (2021). Vitexin Inhibits Protein Glycation through Structural Protection, Methylglyoxal Trapping, and Alteration of Glycation Site. Journal of Agricultural and Food Chemistry, 69 (8), 2462–2476. https://doi.org/10.1021/acs.jafc.0c08052
- Folin, O., Ciocalteu, V. (1927). On tyrosine and tryptophane determinations in proteins. Journal of Biological Chemistry, 73 (2), 627–650. https://doi.org/10.1016/s0021-9258(18)84277-6
- Sánchez-Rangel, J. C., Benavides, J., Heredia, J. B., Cisneros-Zevallos, L., Jacobo-Velázquez, D. A. (2013). The Folin–Ciocalteu assay revisited: improvement of its specificity for total phenolic content determination. Analytical Methods, 5 (21), 5990. https://doi.org/10.1039/c3ay41125g
- Shraim, A. M., Ahmed, T. A., Rahman, M. M., Hijji, Y. M. (2021). Determination of total flavonoid content by aluminum chloride assay: A critical evaluation. LWT, 150, 111932. https://doi.org/10.1016/j.lwt.2021.111932
- Adiotomre, J., Eastwood, M., Edwards, C., Brydon, W. (1990). Dietary fiber: in vitro methods that anticipate nutrition and metabolic activity in humans. The American Journal of Clinical Nutrition, 52 (1), 128–134. https://doi.org/10.1093/ajcn/52.1.128
- Mary Shoba Das, C., Gayathri Devi, S. (2015). In Vitro Glucose Binding Activity of Terminalia Bellirica. Asian Journal of Pharmaceutical and Clinical Research, 8 (2), 320–323.
- Ou, S., Kwok, K., Li, Y., Fu, L. (2001). In Vitro Study of Possible Role of Dietary Fiber in Lowering Postprandial Serum Glucose. Journal of Agricultural and Food Chemistry, 49 (2), 1026–1029. https://doi.org/10.1021/jf000574n
- Poovitha, S., Parani, M. (2016). In vitro and in vivo α-amylase and α-glucosidase inhibiting activities of the protein extracts from two varieties of bitter gourd (Momordica charantia L.). BMC Complementary and Alternative Medicine, 16(S1). https://doi.org/10.1186/s12906-016-1085-1
- McCord, J. M. (2000). The evolution of free radicals and oxidative stress. The American Journal of Medicine, 108 (8), 652–659. https://doi.org/10.1016/s0002-9343(00)00412-5
- Chung, K.-T., Wong, T. Y., Wei, C.-I., Huang, Y.-W., Lin, Y. (1998). Tannins and Human Health: A Review. Critical Reviews in Food Science and Nutrition, 38 (6), 421–464. https://doi.org/10.1080/10408699891274273
- Bhatti, M. Z., Ali, A., Ahmad, A., Saeed, A., Malik, S. A. (2015). Antioxidant and phytochemical analysis of Ranunculus arvensis L. extracts. BMC Research Notes, 8 (1). https://doi.org/10.1186/s13104-015-1228-3
- Vangoori, Y., Dakshinamoorthi, A., Kavimani, S. (2019). Prominent Pancreatic Lipase Inhibition and Free Radical Scavenging Activity of a Myristica fragrans Ethanolic Extract in vitro. Potential Role in Obesity Treatment. Maedica – A Journal of Clinical Medicine, 14 (3). https://doi.org/10.26574/maedica.2019.14.3.254
- Chaput, J.-P., St-Pierre, S., Tremblay, A. (2007). Currently Available Drugs for the Treatment of Obesity: Sibutramine and Orlistat. Mini-Reviews in Medicinal Chemistry, 7 (1), 3–10. https://doi.org/10.2174/138955707779317849
- Klunk, W. E., Jacob, R. F., Mason, R. P. (1999). Quantifying Amyloid β-Peptide (Aβ) Aggregation Using the Congo Red-Aβ (CR–Aβ) Spectrophotometric Assay. Analytical Biochemistry, 266 (1), 66–76. https://doi.org/10.1006/abio.1998.2933
- Lima, M., Baynes, J. W. (2013). Glycation. Encyclopedia of Biological Chemistry. Academic Press, 405–411. https://doi.org/10.1016/b978-0-12-378630-2.00120-1
- Jeyaraj, E. J., Lim, Y. Y., Choo, W. S. (2020). Extraction methods of butterfly pea (Clitoria ternatea) flower and biological activities of its phytochemicals. Journal of Food Science and Technology, 58 (6), 2054–2067. https://doi.org/10.1007/s13197-020-04745-3
- Torres, R. C., Parcon, Ma. R. V., Esmundo, H. J. N., Canillo, D. C. P., Ramil, C. C. (2022). Antioxidant activity and phytochemical constituents of Philippine Clitoria ternatea flowers as a potential therapeutic agent against infectious diseases. Issues in Biological Sciences and Pharmaceutical Research, 10 (2). https://doi.org/10.15739/ibspr.22.003
- Fayed, M. A. A. (2021). Heliotropium; a genus rich in pyrrolizidine alkaloids: A systematic review following its phytochemistry and pharmacology. Phytomedicine Plus, 1 (2), 100036. https://doi.org/10.1016/j.phyplu.2021.100036
- Cajuday, L. A., Membreve, D. M. S., Montealegre, M. V., Serrano, J. E., Baldo, D. E. (2020). In Vitro Antidiabetic, Antiobesity and Antioxidant Activities of Selected Endemic Plants from Mount Mayon and Mount Albay, Philippines. International Journal of Biosciences, 16 (3), 635–648. http://dx.doi.org/10.12692/ijb/16.3.635-648
- Jayasri, M. A., Gunasekaran, S., Radha, A., Mathew, T. L. (2008). Anti-diabetic effect of Costus pictus leaves in normal and streptozotocin-induced diabetic rats. International Journal of Diabetes and Metabolism, 16 (3), 117–122. https://doi.org/10.1159/000497662
- Ibrahim, S. R. M., Bagalagel, A. A., Diri, R. M., Noor, A. O., Bakhsh, H. T., Mohamed, G. A. (2022). Phytoconstituents and Pharmacological Activities of Indian Camphorweed (Pluchea indica): A Multi-Potential Medicinal Plant of Nutritional and Ethnomedicinal Importance. Molecules, 27 (8), 2383. https://doi.org/10.3390/molecules27082383
- Chagas, M. do S. S., Behrens, M. D., Moragas-Tellis, C. J., Penedo, G. X. M., Silva, A. R., Gonçalves-de-Albuquerque, C. F. (2022). Flavonols and Flavones as Potential anti-Inflammatory, Antioxidant, and Antibacterial Compounds. Oxidative Medicine and Cellular Longevity, 2022, 1–21. https://doi.org/10.1155/2022/9966750
- Pathak, M. P., Pathak, K., Saikia, R., Gogoi, U., Patowary, P., Chattopadhyay, P., Das, A. (2023). Therapeutic potential of bioactive phytoconstituents found in fruits in the treatment of non-alcoholic fatty liver disease: A comprehensive review. Heliyon, 9 (4), e15347. https://doi.org/10.1016/j.heliyon.2023.e15347
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Aaron L. Degamon, Misshell L. Lavilla, Orlie B. Basalo, Nesyl Mae O. Butong, Danical Necole P. Cabural, Maria Angelika M. Villarosa, Anelyn P. Bendoy, Charlie Jr. A. Lavilla

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






