Spectrophotometric determination of nimodipine, nitrendipine, lacidipine in tablets via derivatization with para-dimethylaminobenzaldehyde
DOI:
https://doi.org/10.15587/2519-4852.2025.332156Keywords:
calcium channel blockers, nitrendipine, nimodipine, lacidipine, spectrophotometry, para-dimethylaminobenzaldehyde, assay, validationAbstract
The proposed approach involves the interaction of NIM, NIT, and LAC directly with DABA reagent to produce coloured products with its further spectrophotometric analysis by the development of simple, available, and alternative spectrophotometric methods.
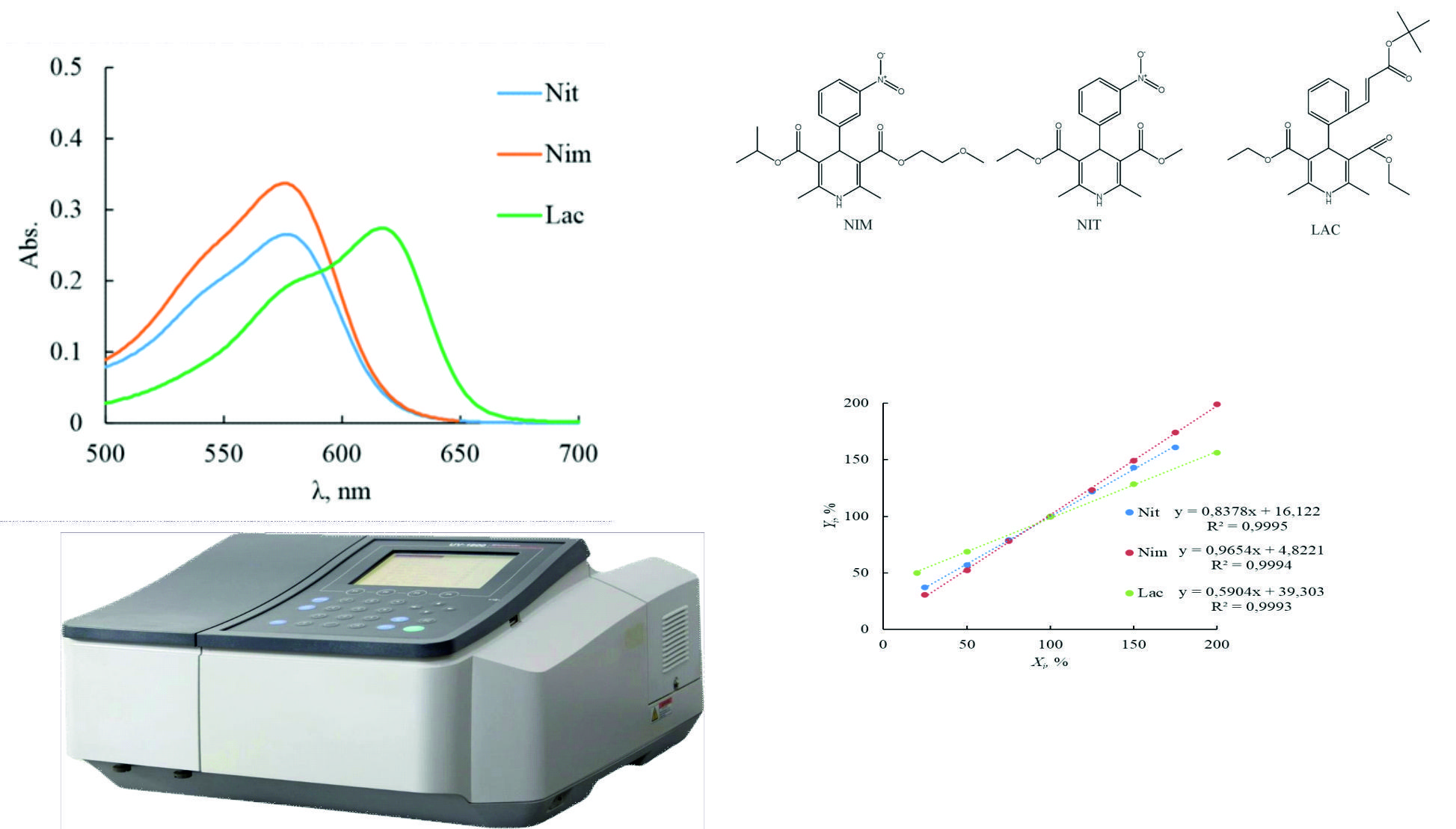
Material and methods: UV-visible double beam spectrophotometer Shimadzu UV-1800 (Japan) with included UV-Probe version 2.62 software was employed. Additional equipment included a precise analytical balance RAD WAG AS 200/C (Poland), an ultrasonic bath Elmasonic EASY 60H with a frequency of 40 kHz and a water bath VB-4 were used in the developed procedure. LAC, NIM and NIT (purity ≥98% (HPLC)) were supplied from Sigma-Aldrich Chemicals Co. (St. Louis, MO, USA). NIT 10 mg tablets, NIM 30 mg tablets were purchased from local drugstore. LAC 2 mg tablets were purchased from europharm.com.ua.
Methanol was produced by Honeywell and had a purity of 99.9%. HCl conc. (fuming, by Sigma Aldrich was used. DABA utilized in the experiment was analytical grade.
Results and discussion: Simple, available and alternative visible spectrophotometric methods for the determination of nitrendipine (NIT), nimodipine (NIM), and lacidipine (LAC) in tablets through derivatization with the para-dimethylaminobenzaldehyde (DABA) have been developed. The optimal parameters for CCBs spectrophotometric analysis were as follows: detection wavelength - 577 nm for NIM, NIT and 616 nm for LAC, concentrated hydrochloric acid, 1.5 mL of 0.1% DABA solution, 15 min boiling at 100 °C. The concentration was linearly proportional to absorbance values in the range of 25 – 175 μg/mL (NIT), 25 – 200 μg/mL (NIM), 20 – 200 μg/mL (LAC). Estimation of LOD and LOQ parameters were obtained as 3.75 μg/mL and 11.38 μg/mL (NIT), 4.43 μg/mL and 13.43 μg/mL (NIM), 6.30μg/ml and 19.1μg/mL (LAC).
Conclusions. In this work, thorough scientific research was carried out with the presentation of the method of selection of the optimal reaction conditions and spectrophotometric methods for determining NIT, NIM, and LAC in tablets were developed. In addition, the three studied CCBs were quantified using easy-to-implement, simple, cost-effective spectrophotometric approaches. The proposed methods can be used as alternatives and arbitrage, which significantly expands the bank of analytical methods. Moreover, the described methods can be easily implemented for routine pharmaceutical analysis
Supporting Agency
- The research leading to these results has received funding from the Ministry of Health of Ukraine, under the project number 0124U000065.
References
- Hypertension. World Health Organization. Available at: https://www.who.int/health-topics/hypertension#tab Last accessed: 31. 05. 2024
- McKeever, R. G., Patel, P., Hamilton, R. J. (2024). Calcium Channel Blockers. Treasure Island: StatPearls Publishing. Available at: https://www.ncbi.nlm.nih.gov/books/NBK482473/
- Lin, Y., Ma, L. (2018). Blood pressure lowering effect of calcium channel blockers on perioperative hypertension. Medicine, 97 (48), e13152. https://doi.org/10.1097/md.0000000000013152
- Pavasini, R., Camici, P. G., Crea, F., Danchin, N., Fox, K., Manolis, A. J. et al. (2019). Anti-anginal drugs: Systematic review and clinical implications. International Journal of Cardiology, 283, 55–63. https://doi.org/10.1016/j.ijcard.2018.12.008
- Shadman, J., Panahpour, H., Alipour, M. R., Salimi, A., Shahabi, P., Azar, S. S. (2024). Investigating the therapeutic effects of nimodipine on vasogenic cerebral edema and blood-brain barrier impairment in an ischemic stroke rat model. Neuropharmacology, 257. https://doi.org/10.1016/j.neuropharm.2024.110054
- Nimodipine. USP Monographs. Available at: https://doi.usp.org/USPNF/USPNF_M56650_05_01.html Last accessed: 31.05.2024
- The European Pharmacopoeia (2022). European Directorate for the Quality of Medicines & HealthCare. Available at: https://www.edqm.eu/en/european-pharmacopoeia-ph.-eur.-11th-edition
- Isse, F. A., Le, T., Mahmoud, S. H. (2021). Enantioselective assay of nimodipine in human plasma using liquid chromatography–tandem mass spectrometry. Biomedical Chromatography, 35 (2). https://doi.org/10.1002/bmc.4971
- Mohamed, S., Riva, R., Contin, M. (2016). Simple and validated UHPLC–MS/MS analysis of nimodipine in plasma and cerebrospinal fluid of patients with subarachnoid haemorrhage. Journal of Chromatography B, 1028, 94–99. https://doi.org/10.1016/j.jchromb.2016.06.012
- Riekes, M. K., Rauber, G. S., Kuminek, G., Tagliari, M. P., Cardoso, S. G., Stulzer, H. K. (2013). Determination of Nimodipine in the Presence of its Degradation Products and Overall Kinetics through a Stability-Indicating LC Method. Journal of Chromatographic Science, 51 (6), 511–516. https://doi.org/10.1093/chromsci/bms174
- Feng, X. U., Xiaoxue, L. I., Li, L. I., Jicheng, L. U. (2018). Rapid detection of three chemical components added illegally into antihypertensive health food by TLC concentration in situ combined with Micro-Raman spectroscopy method. Chemical journal of Chinese Universities, 39 (6), 1172. https://doi.org/10.7503/cjcu20170735
- Wang, W., Li, P., Fang, M., Li, X., Zhang, Y., Zhang, T. (2020). SFC-MS/MS Method for Simultaneous Determination of Nimodipine and 3-n-Butylphthalide in Beagle Plasma: Application to Pharmacokinetic Interaction Study. Bioanalysis, 12 (21), 1509–1519. https://doi.org/10.4155/bio-2020-0229
- Galvão, A. F., Ajimura, T. O., Aguiar, F. A., Borges, K. B., Masetto de Gaitani, C. (2012). Stability-indicating methods for the enantioselective determination of dihydropyridines by high performance liquid chromatography and capillary electrophoresis. Analytical Methods, 4 (9), 2953. https://doi.org/10.1039/c2ay25055a
- Lacidipine. PubChem. National Library of Medicine. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/5311217 Last accessed: 31.05.2024
- Liu, X., Huang, Z., Zhang, Y., Shui, X., Liu, F., Wu, Z. et al. (2021). Lacidipine Ameliorates the Endothelial Senescence and Inflammatory Injury Through CXCR7/P38/C/EBP-β Signaling Pathway. Frontiers in Cardiovascular Medicine, 8. https://doi.org/10.3389/fcvm.2021.692540
- Belal, F., Sharaf EL‐Din, M., Tolba, M. M., Alaa, H. (2015). Micelle‐enhanced spectrofluorimetric method for determination of lacidipine in tablet form; application to content uniformity testing. Luminescence, 30 (6), 805–811. https://doi.org/10.1002/bio.2823
- Shen, J., Hu, A., Yang, Y., Nie, T., Huang, S., Cheng, Z. et al. (2024). Ternary solid dispersions of lacidipine: Enhancing dissolution and supersaturation maintenance through strategic formulation optimization. International Journal of Pharmaceutics, 654. https://doi.org/10.1016/j.ijpharm.2024.123989
- Preeti, Sambhakar, S., Malik, R., Bhatia, S., Harrasi, A. A., Saharan, R., Aggarwal, G. et al. (2024). Lipid Horizons: Recent Advances and Future Prospects in LBDDS for Oral Administration of Antihypertensive Agents. International Journal of Hypertension, 1–54. https://doi.org/10.1155/2024/2430147
- Dandu, G., Akkimi, P., Thirupathireddy, A., Venkateswara, B. (2019). Analytical method development and validation for the estimation of lacidipine in bulk and dosage form by RP-HPLC. Asian Journal of Chemical and Pharmaceutical Research, 7 (1), 1–6.
- Sai Chebrolu, T., Kumar, L., Verma, R. (2021). Lacidipine: Review of Analytical Methods Developed for Pharmaceutical Dosage forms and Biological Fluids. Bioanalysis, 13 (12), 1011–1024. https://doi.org/10.4155/bio-2021-0024
- Kisan Chatki, P., Tabassum, S. (2021). Analytical Methods of Dihydropyridines Based Calcium Channel Blockers - Amlodipine, Lacidipine, Isradipine, Nifedipine, Felodipine, Cilnidipine and its related formulations: A Review. Asian Journal of Research in Chemistry, 221–234. https://doi.org/10.52711/0974-4150.2021.00039
- Chen, H., Li, L., Song, S., Du, Y., Jin, X., Ding, P. et al. (2018). Determination of lacidipine in human plasma by LC-MS/MS: Application in a bioequivalence study. Int. Journal of Clinical Pharmacology and Therapeutics, 56 (10), 493–500. https://doi.org/10.5414/cp203250
- Nitrendipine. PubChem. National Library of Medicine. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/Nitrendipine Last accessed: 31.05.2024
- Yusupova, K. H. F., Abdullaeva, G. J., Khamidullaeva, G. A., Ibrohimov, N. N. (2023). Neuroprotective efficacy of combined antihypertensive treatment with the inclusion of nitrendipine in patients with arterial hypertension. European Heart Journal, 44 (2). https://doi.org/10.1093/eurheartj/ehad655.2340
- Mohammad, M. A.-A., Mahrouse, M. A., Amer, E. A. H., Elharati, N. S. (2021). Simultaneous determination of enalapril maleate and nitrendipine in tablets using spectrophotometric methods manipulating ratio spectra. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 244, 118894. https://doi.org/10.1016/j.saa.2020.118894
- Sharma, A., Sharma, R., Singh Bora, K., Harikumar, S. L. (2022). Pharmacokinetic investigation of nitrendipine encapsulated niosomal gel in rat plasma by RP-HPLC method. Materials Today: Proceedings, 68 (4), 653–657. https://doi.org/10.1016/j.matpr.2022.05.301
- Kawabata, K., Muraoka, H., Miyara, M., Kotake, Y., Nishi, H. (2023). Photodegradation profiling of nitrendipine: evaluation of active pharmaceutical ingredient, tablets and its altered forms. Analytical Sciences, 39 (11), 1791–1803. https://doi.org/10.1007/s44211-023-00400-x
- Fares, H., DiNicolantonio, J. J., O’Keefe, J. H., Lavie, C. J. (2016). Amlodipine in hypertension: a first-line agent with efficacy for improving blood pressure and patient outcomes. Open Heart, 3 (2), e000473. https://doi.org/10.1136/openhrt-2016-000473
- Li, X., Wang, C., Li, T., Liu, Y., Liu, S., Tao, Y. et al. (2020). Bioequivalence of levamlodipine besylate tablets in healthy Chinese subjects: a single-dose and two-period crossover randomized study. BMC Pharmacology and Toxicology, 21 (1). https://doi.org/10.1186/s40360-020-00459-6
- Kryskiw, L., Horyn, M., Kucher, T., Zarivna, N., Poliak, O., Logoyda, L. (2024). Novel eco-friendly spectrophotometric determination of lercanidipine hydrochloride in tablets using methyl red. ScienceRise: Pharmaceutical Science, 3 (49), 47–53. https://doi.org/10.15587/2519-4852.2024.307263
- Nguyen, T. D., Le, T. H., Nguyen, T. H., Hoang, T. T. M. (2022). Extractive spectrophotometric determination of nimodipine through ion-pair complex formation with bromothymol blue. Journal of Science and Technique, 17 (1), 5–16. https://doi.org/10.56651/lqdtu.jst.v17.n01.297
- Alkhalil, R., Attal, A., Sakur, A. A. (2019). Spectrophotometric Determination of Amlodipine Besylate in Pure Form and Pharmaceutical Formulation using Amido Black. Research Journal of Pharmacy and Technology, 12 (7), 3389–3392. https://doi.org/10.5958/0974-360x.2019.00572.9
- Abed, S. S. (2020). Cloud-Point Extraction and Spectrophotometric Determination of Nifedipine in Pharmaceutical Dosage Forms. Systematic Reviews in Pharmacy, 11 (7), 125–130.
- Tulasamma, P., Venkateswarlu, P. (2016). Spectrophotometric determination of nifedipine in pharmaceutical formulations, serum and urine samples via oxidative coupling reaction. Arabian Journal of Chemistry, 9, S1603–S1609. https://doi.org/10.1016/j.arabjc.2012.04.025
- Reddy, M. N., Murthy, T. K., Rao Kanna, K. V., Gopal Hara, A. V., Sankar, D. G. (2001). New spectrophotometric methods for the determination of nimodipine. Indian Drugs, 38 (3), 140–142.
- Moorthi, C., Aju, M., Elsadig, Prezzy, Sumithira, G. (2011). Spectrophotometric determination of lacidipine in bulk and tablet dosage form. Journal of Applied Pharmaceutical Science, 1 (5), 209–213.
- Gudavarthi, S. (2012). Rajiv Gandhi University of Health Sciences (India) ProQuest Dissertations & Theses.
- Hamd, M. A. E., Derayea, S. M., Abdelmageed, O. H., Askal, H. F. (2013). A Novel Spectrophotometric Method for Determination of Five 1,4-Dihydropyridine Drugs in Their Tablets and Capsules Using Vanillin Reagent. American Journal of Analytical Chemistry, 4 (3), 148–157. https://doi.org/10.4236/ajac.2013.43020
- Marzouq, M. A., El Hamd, M. A., Ahmed, S. A., Askal, H. F., Saleh, G. A. (2015). Spectrophotometric determination of some 1,4-dihydropyridine drugs in their pharmaceutical preparations and spiked human plasma. Der Pharma Chemica, 7 (8), 105–111.
- - (diethylamino) benzaldehyde. PubChem. National Library of Medicine. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/7479 Last accessed: 31.05.2024
- Mohamed, S., Yousif, E., Kadhom, M., Bufaroosha, M. (2024). Enhanced photostability of polyvinyl chloride films through antipyrine derivatives: a comprehensive study on UV resistance and degradation inhibition. Macromolecular Research, 33 (1), 85–103. https://doi.org/10.1007/s13233-024-00310-5
- Validation of Analytical Procedures Q2 (R2) (2023). ICH. Available at: https://database.ich.org/sites/default/files/ICH_Q2%28R2%29_Guideline_2023_1130.pdf Last accessed: 24.06.2024
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Liliya Logoyda, Mariana Horyn, Liubomyr Kryskiw, Tetyana Kucher, Nadiya Zarivna, Nataliia Shulyak, Iryna Ivanchuk

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






