Pharmacological evaluation and potential epigenetic modulation of a zinc-cysteine complex for type 2 diabetes therapy
DOI:
https://doi.org/10.15587/2519-4852.2025.338156Keywords:
Type 2 Diabetes, zinc-cysteine complex, AGEs, DNA methylation, molecular dockingAbstract
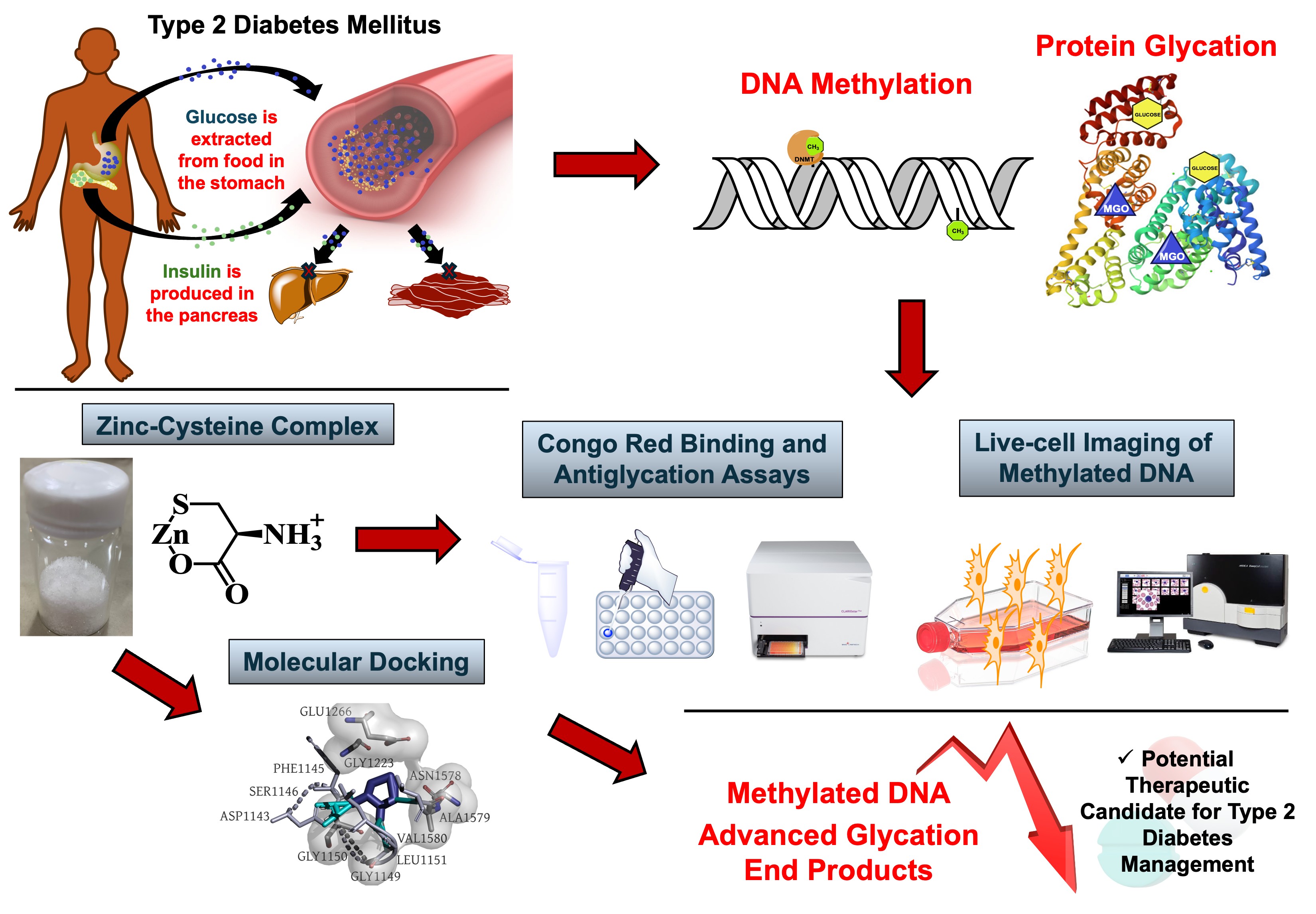
Type 2 Diabetes (T2D) is a complex metabolic disorder that involves more than just glucose imbalance. Protein glycation, and epigenetic dysregulation—particularly aberrant DNA methylation—play critical roles in the onset and progression of the disease. However, current therapies remain limited in directly targeting these underlying molecular mechanisms.
The aim. This study investigated a zinc-monocysteine complex (ZMC) as a potential multi-target therapeutic candidate for T2D, exploring its novel application in modulating protein glycation and DNA methylation events.
Materials and methods. The structural integrity of ZMC was confirmed through NMR, FT-IR, UV-visible, CHN analysis, and powder XRD techniques. In vitro assays compared ZMC and unbound L-cysteine (CYS) for their abilities to inhibit advanced glycation end-products (AGEs) and preserved protein secondary structure under glycation stress, using BSA-glucose and methylglyoxal (MGO) model systems. To support potential epigenetic modulation, molecular docking studies were conducted to evaluate the interaction of ZMC with DNA methyltransferase, DNMT1. Live-cell imaging was performed on C2C12 and HEK293T cells to assess changes in methylation-associated signals following ZMC treatment.
Results. ZMC was defined structurally as a 1:1 amorphous cyclic salt. It outperformed CYS in inhibiting AGE formation at 5 mM (BSA-glucose) and 1 mM (BSA-MGO). It also better preserved protein secondary structure at 5 mM (BSA-glucose) and 10 mM (BSA-MGO). Although docking suggested limited affinity for DNA methyltransferase (DNMT1: -5.1 kcal/mol) , live-cell imaging indicated reduced methylation-associated signals in especially in C2C12 cells following treatment.
Conclusion. Together, ZMC demonstrates multi-target potential in addressing key metabolic and epigenetic factors involved in T2D. Its protective effects are primarily attributed to metabolic regulation. These findings support the continued development of ZMC as a promising scaffold for future T2D therapeutics
Supporting Agency
- This research was conducted with funding from Department of Science and Technology-Accelerated Science and Technology Human Resource Development Program (DOST-ASTHRDP). The primary author is also a recipient of the DOST-ASTHRDP Research Enrichment “Sandwich” Program which allowed him to perform experiments in Niigata University, Japan as a visiting researcher.
References
- Sun, H., Saeedi, P., Karuranga, S., Pinkepank, M., Ogurtsova, K., Duncan, B. B. et al. (2022). IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Research and Clinical Practice, 183, 109119. https://doi.org/10.1016/j.diabres.2021.109119
- Five questions on the IDF Diabetes Atlas (2013). Diabetes Research and Clinical Practice, 102 (2), 147–148. https://doi.org/10.1016/j.diabres.2013.10.013
- Zhou, B., Rayner, A. W., Gregg, E. W., Sheffer, K. E., Carrillo-Larco, R. M., Bennett, J. E. et al. (2024). Worldwide trends in diabetes prevalence and treatment from 1990 to 2022: a pooled analysis of 1108 population-representative studies with 141 million participants. The Lancet, 404 (10467), 2077–2093. https://doi.org/10.1016/s0140-6736(24)02317-1
- Cando, L. F. T., Quebral, E. P. B., Ong, E. P., Catral, C. D. M., Relador, R. J. L., Velasco, A. J. D. et al. (2024). Current status of diabetes mellitus care and management in the Philippines. Diabetes & Metabolic Syndrome: Clinical Research & Reviews, 18 (2), 102951. https://doi.org/10.1016/j.dsx.2024.102951
- Jiang, J., Zhao, C., Han, T., Shan, H., Cui, G., Li, S., Xie, Z., Wang, J. (2022). Advanced Glycation End Products, Bone Health, and Diabetes Mellitus. Experimental and Clinical Endocrinology & Diabetes, 130 (10), 671–677. https://doi.org/10.1055/a-1861-2388
- Uceda, A. B., Mariño, L., Casasnovas, R., Adrover, M. (2024). An overview on glycation: molecular mechanisms, impact on proteins, pathogenesis, and inhibition. Biophysical Reviews, 16 (2), 189–218. https://doi.org/10.1007/s12551-024-01188-4
- Ishrat, N., Khan, H., Patel, O. P. S., Mahdi, A. A., Mujeeb, F., Ahmad, S. (2021). Role of Glycation in Type 2 Diabetes Mellitus and Its Prevention through Nymphaea Species. BioMed Research International, 2021 (1). https://doi.org/10.1155/2021/7240046
- Kim, M. (2019). DNA methylation: a cause and consequence of type 2 diabetes. Genomics & Informatics, 17 (4), e38. https://doi.org/10.5808/gi.2019.17.4.e38
- Rönn, T., Ling, C. (2015). DNA Methylation as a Diagnostic and Therapeutic Target in the Battle Against Type 2 Diabetes. Epigenomics, 7 (3), 451–460. https://doi.org/10.2217/epi.15.7
- Kaimala, S., Ansari, S. A., Emerald, B. S. (2023). DNA methylation in the pathogenesis of type 2 diabetes. Hormones and Epigenetics. Elsevier Inc., 147–169. https://doi.org/10.1016/bs.vh.2022.11.002
- Chong, K., Chang, J. K., Chuang, L. (2024). Recent advances in the treatment of type 2 diabetes mellitus using new drug therapies. The Kaohsiung Journal of Medical Sciences, 40 (3), 212–220. https://doi.org/10.1002/kjm2.12800
- Maanvizhi, S., Boppana, T., Krishnan, C., Arumugam, G. (2014). Metal complexes in the management of diabetes mellitus: A new therapeutic strategy. International Journal of Pharmacy and Pharmaceutical Science, 6, 40–44. Available at: https://journals.innovareacademics.in/index.php/ijpps/article/view/1778/10461
- Matsukura, T., Tanaka, H. (2000). Applicability of Zinc Complex of L-Carnosine for Medical Use. Biochemistry, 65 (7), 817–823. Available at: https://pubmed.ncbi.nlm.nih.gov/10951100/
- Tate, D. J., Newsome, D. A. (2006). A Novel Zinc Compound (Zinc Monocysteine) Enhances the Antioxidant Capacity of Human Retinal Pigment Epithelial Cells. Current Eye Research, 31 (7-8), 675–683. https://doi.org/10.1080/02713680600801024
- Tate, D. J., Newsome, D. A. (2007). Preparation of a Zinc Monocysteine Compound. Synthetic Communications, 37 (6), 909–914. https://doi.org/10.1080/00397910601163612
- Miroliaei, M., Khazaei, S., Moshkelgosha, S., Shirvani, M. (2011). Inhibitory effects of Lemon balm (Melissa officinalis, L.) extract on the formation of advanced glycation end products. Food Chemistry, 129 (2), 267–271. https://doi.org/10.1016/j.foodchem.2011.04.039
- Ni, M., Song, X., Pan, J., Gong, D., Zhang, G. (2021). Vitexin Inhibits Protein Glycation through Structural Protection, Methylglyoxal Trapping, and Alteration of Glycation Site. Journal of Agricultural and Food Chemistry, 69 (8), 2462–2476. https://doi.org/10.1021/acs.jafc.0c08052
- Hori, Y., Otomura, N., Nishida, A., Nishiura, M., Umeno, M., Suetake, I., Kikuchi, K. (2018). Synthetic-Molecule/Protein Hybrid Probe with Fluorogenic Switch for Live-Cell Imaging of DNA Methylation. Journal of the American Chemical Society, 140 (5), 1686–1690. https://doi.org/10.1021/jacs.7b09713
- Brabha, M. J., Malbi, M. A. (2023). Synthesis, characterization and biological activity of zinc complexes of ethylenediamine and its derivatives. Chemical Physics Impact, 7, 100248. https://doi.org/10.1016/j.chphi.2023.100248
- Campos, A. F. C., Reis, P. F., Neiva, J. V. C. M., Guerra, A. A. A. M., Kern, C., Silva, M. F. P. da et al. (2021). Reusable cysteine-ferrite-based magnetic nanopowders for removal of lead ions from water. Materials Research, 24 (5). https://doi.org/10.1590/1980-5373-mr-2021-0217
- Soomro, R. A., Nafady, A., Sirajuddin, Memon, N., Sherazi, T. H., Kalwar, N. H. (2014). l-cysteine protected copper nanoparticles as colorimetric sensor for mercuric ions. Talanta, 130, 415–422. https://doi.org/10.1016/j.talanta.2014.07.023
- Stark, F., Loderer, C., Petchey, M., Grogan, G., Ansorge‐Schumacher, M. B. (2022). Advanced Insights into Catalytic and Structural Features of the Zinc‐Dependent Alcohol Dehydrogenase from Thauera aromatica. ChemBioChem, 23 (15). https://doi.org/10.1002/cbic.202200149
- Khan, M. M., Kalathil, S., Lee, J.-T., Cho, M.-H. (2012). Synthesis of Cysteine Capped Silver Nanoparticles by Electrochemically Active Biofilm and their Antibacterial Activities. Bulletin of the Korean Chemical Society, 33(8), 2592–2596. https://doi.org/10.5012/bkcs.2012.33.8.2592
- Kieninger, M., Ventura, O. N. (2009). On the structure, infrared and Raman spectra of the 2:1 cysteine–Zn complex. Theoretical Chemistry Accounts, 125 (3-6), 279–291. https://doi.org/10.1007/s00214-009-0697-7
- Guo, T., Xu, J., Fan, Z., Du, Y., Pan, Y., Xiao, H. et al. (2019). Preparation and characterization of cysteine‐formaldehyde cross‐linked complex for CO2 capture. The Canadian Journal of Chemical Engineering, 97 (12), 3012–3024. https://doi.org/10.1002/cjce.23595
- Zerner, M. C., Loew, G. H., Kirchner, R. F., Mueller-Westerhoff, U. T. (1980). An intermediate neglect of differential overlap technique for spectroscopy of transition-metal complexes. Ferrocene. Journal of the American Chemical Society, 102 (2), 589–599. https://doi.org/10.1021/ja00522a025
- Han, J. (2010). Vibrational and Electronic Spectroscopic Characterizations of Amino Acid-Metal Complexes. Journal of the Korean Society for Applied Biological Chemistry, 53 (6), 821–825. https://doi.org/10.3839/jksabc.2010.124
- Tripathi, I. P., Dwivedi, A., Mishra, M. K. (2019). Synthesis and Characterization of Some Zn (II) Complexes of L-Glutamic Acid and L-Aspartic Acid. International Journal of Advanced Scientific Research and Management, 5, 153–159. Available at: https://ijasrm.com/wp-content/uploads/2019/05/IJASRM_V4S4_1317_153_159.pdf
- Trampuž, M., Žnidarič, M., Gallou, F., Časar, Z. (2022). Does the Red Shift in UV–Vis Spectra Really Provide a Sensing Option for Detection of N-Nitrosamines Using Metalloporphyrins? ACS Omega, 8 (1), 1154–1167. https://doi.org/10.1021/acsomega.2c06615
- Timón, V., Maté, B., Herrero, V. J., Tanarro, I. (2021). Infrared spectra of amorphous and crystalline urea ices. Physical Chemistry Chemical Physics, 23 (39), 22344–22351. https://doi.org/10.1039/d1cp03503g
- Kheshtzar, R., Berenjian, A., Taghizadeh, S.-M., Ghasemi, Y., Asad, A. G., Ebrahiminezhad, A. (2019). Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles. Green Processing and Synthesis, 8 (1), 846–855. https://doi.org/10.1515/gps-2019-0055
- Nazir, S., Anwar, J., Munawar, M. A., Best, S. P., Cheah, M. (2016). Transition Metal Complexes of S-Propyl- L -Cysteine. Journal of The Chemical Society of Pakistan, 38, 415–423. Available at: https://jcsp.org.pk/PublishedVersion/67f48146-16cf-4d24-bfe5-a74da9c85acfManuscript%20no%2006,%20Final%20Gally%20Proof%20of%2010829%20_Shahbaz%20Na.pdf
- Yang, Y., Engkvist, O., Llinàs, A., Chen, H. (2012). Beyond Size, Ionization State, and Lipophilicity: Influence of Molecular Topology on Absorption, Distribution, Metabolism, Excretion, and Toxicity for Druglike Compounds. Journal of Medicinal Chemistry, 55 (8), 3667–3677. https://doi.org/10.1021/jm201548z
- Lavilla, M. L., Lavilla, C. J. A., Burnea, F. K. B., Inutan, E. D. (2024). L-cysteine sequestering methyl glyoxal prevents protein glycation: a combined in vitro and in silico evaluation. Current Issues in Pharmacy and Medical Sciences, 37 (2), 114–120. https://doi.org/10.2478/cipms-2024-0019
- Tarwadi, K. V., Agte, V. V., Kelkar, A. R. (2018). Influence of Selected Micronutrients on Glycation of Human Lens Proteins: Implications in Diabetic Cataract. Acta Scientific Ophthalmology, 1 (2), 4–10. Available at: https://actascientific.com/ASOP/pdf/ASOP-01-0009.pdf
- Leyder, T., Mignon, J., Mottet, D., Michaux, C. (2022). Unveiling the Metal-Dependent Aggregation Properties of the C-terminal Region of Amyloidogenic Intrinsically Disordered Protein Isoforms DPF3b and DPF3a. International Journal of Molecular Sciences, 23 (23), 15291. https://doi.org/10.3390/ijms232315291
- Tupe, R., Kulkarni, A., Adeshara, K., Sankhe, N., Shaikh, S., Dalal, S. et al. (2015). Zinc inhibits glycation induced structural, functional modifications in albumin and protects erythrocytes from glycated albumin toxicity. International Journal of Biological Macromolecules, 79, 601–610. https://doi.org/10.1016/j.ijbiomac.2015.05.028
- Moulahoum, H., Ghorbanizamani, F., Timur, S., Zihnioglu, F. (2020). Zinc enhances carnosine inhibitory effect against structural and functional age-related protein alterations in an albumin glycoxidation model. BioMetals, 33 (6), 353–364. https://doi.org/10.1007/s10534-020-00254-0
- Pace, N., Weerapana, E. (2014). Zinc-Binding Cysteines: Diverse Functions and Structural Motifs. Biomolecules, 4 (2), 419–434. https://doi.org/10.3390/biom4020419
- Holendova, B., Plecita-Hlavata, L. (2023). Cysteine residues in signal transduction and its relevance in pancreatic beta cells. Frontiers in Endocrinology, 14. https://doi.org/10.3389/fendo.2023.1221520
- Raciti, G. A., Desiderio, A., Longo, M., Leone, A., Zatterale, F., Prevenzano, I. et al. (2021). DNA Methylation and Type 2 Diabetes: Novel Biomarkers for Risk Assessment? International Journal of Molecular Sciences, 22 (21), 11652. https://doi.org/10.3390/ijms222111652
- Cassandri, M., Smirnov, A., Novelli, F., Pitolli, C., Agostini, M., Malewicz, M. et al. (2017). Zinc-finger proteins in health and disease. Cell Death Discovery, 3 (1). https://doi.org/10.1038/cddiscovery.2017.71
- Noronha, N. Y., Barato, M., Sae-Lee, C., Pinhel, M. A. de S., Watanabe, L. M., Pereira, V. A. B. et al. (2022). Novel Zinc-Related Differentially Methylated Regions in Leukocytes of Women With and Without Obesity. Frontiers in Nutrition, 9. https://doi.org/10.3389/fnut.2022.785281
- Zhang, H.-H., Han, X., Wang, M., Hu, Q., Li, S., Wang, M., Hu, J. (2019). The Association between Genomic DNA Methylation and Diabetic Peripheral Neuropathy in Patients with Type 2 Diabetes Mellitus. Journal of Diabetes Research, 2019, 1–9. https://doi.org/10.1155/2019/2494057
- Wang, X., Yang, W., Zhu, Y., Zhang, S., Jiang, M., Hu, J., Zhang, H.-H. (2022). Genomic DNA Methylation in Diabetic Chronic Complications in Patients With Type 2 Diabetes Mellitus. Frontiers in Endocrinology, 13. https://doi.org/10.3389/fendo.2022.896511
- Hafez, S. M., Abou-Youssef, Hazem. E.-S., Awad, M. A.-K., Kamel, S. A., Youssef, R. N., Elshiekh, S. M. et al. (2021). Insulin-like growth factor binding protein 1 DNA methylation in type 2 diabetes. Egyptian Journal of Medical Human Genetics, 22 (1). https://doi.org/10.1186/s43042-021-00153-0
- Willmer, T., Johnson, R., Louw, J., Pheiffer, C. (2018). Blood-Based DNA Methylation Biomarkers for Type 2 Diabetes: Potential for Clinical Applications. Frontiers in Endocrinology, 9. https://doi.org/10.3389/fendo.2018.00744
- Cheng, Y., Gadd, D. A., Gieger, C., Monterrubio-Gómez, K., Zhang, Y., Berta, I. et al. (2023). Development and validation of DNA methylation scores in two European cohorts augment 10-year risk prediction of type 2 diabetes. Nature Aging, 3 (4), 450–458. https://doi.org/10.1038/s43587-023-00391-4
- Ahmed, N. (2005). Advanced glycation endproducts – role in pathology of diabetic complications. Diabetes Research and Clinical Practice, 67 (1), 3–21. https://doi.org/10.1016/j.diabres.2004.09.004
- Bulahan, G., Lavilla, C. (2025). Zinc-Cysteine Coupling Demonstrates Potent In Vitro Antioxidant Activity and Preserves Cell Viability Under Glucolipotoxicity-Induced Oxidative Stress. International Journal of Scientific Engineering and Science, 9 (5), 182–185. Available at: https://ijses.com/wp-content/uploads/2025/05/78-IJSES-V9N5.pdf
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Godzelle Ogoc Bulahan, Orlie B. Basalo, Hajime Iwamoto, Aaron L. Degamon, James V. Lavilla Jr., Richemae Grace R. Lebosada, Charlie A. Lavilla Jr.

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






