Study of some properties of hydrophilic ointment bases depending on their composition
DOI:
https://doi.org/10.15587/2519-4852.2025.339597Keywords:
hydrophilic base, ointment, rheological parameter, absorption, release, EPR spectrum parameter, growth inhibition zoneAbstract
The aim. To study the properties of multicomponent hydrophilic ointment bases.
Materials and methods. The study focused on hydrophilic bases with varying formulations and ointments containing ofloxacin. The rheological properties of the bases were studied using rotational viscometry, and water absorption and ofloxacin release were investigated using diffusion through a semipermeable membrane. The water content was determined by the semi-micro method, and the ofloxacin content was determined by liquid chromatography. Four spin probes were utilized in the experiment, and the EPR spectra of these spin probes in a mixed solvent PG – M400 and bases were obtained. The type and parameters of the EPR spectra were evaluated. Surface tension was measured using the maximum bubble pressure method, and antibacterial activity was assessed by the agar diffusion method.
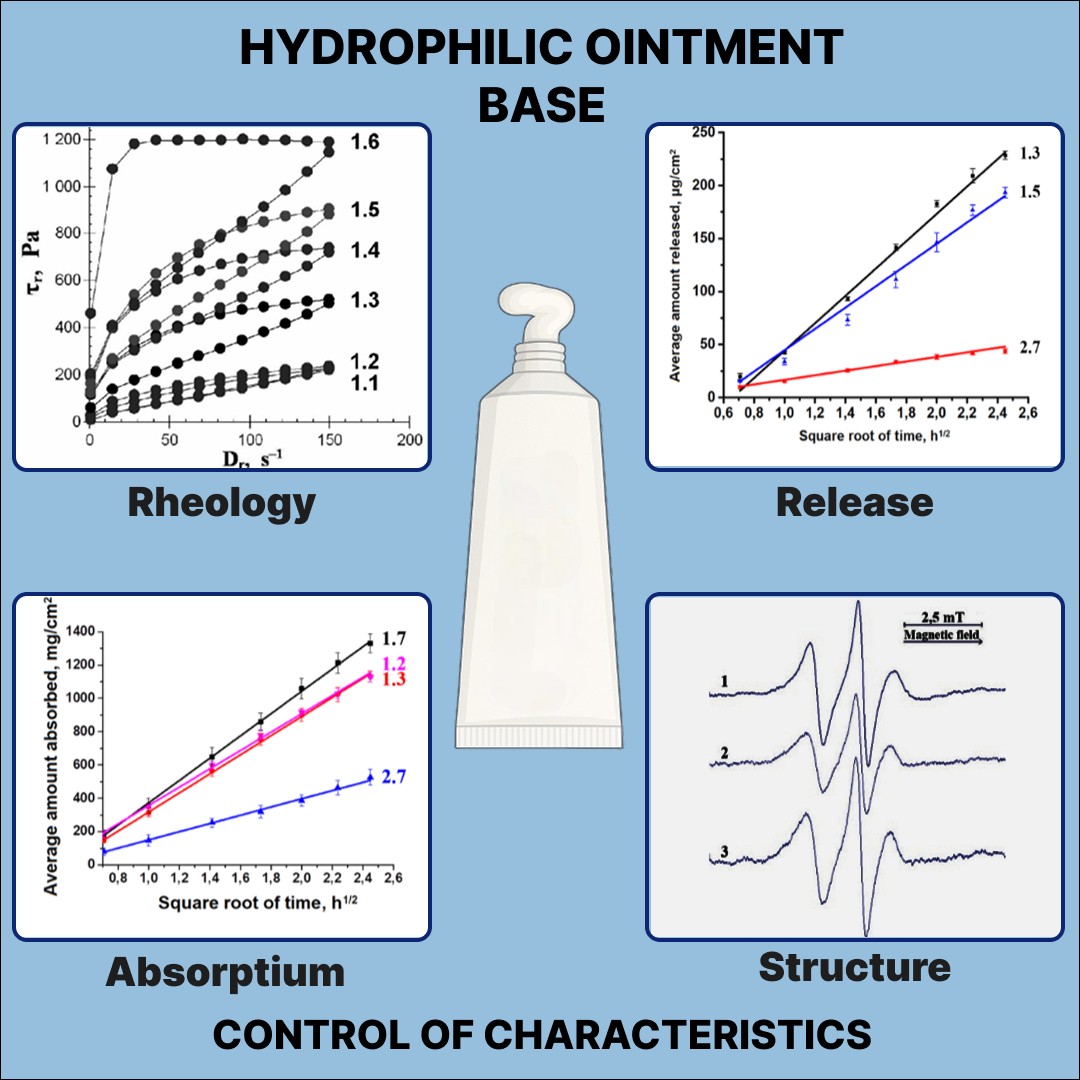
Results. The rheological parameters of hydrophilic bases are contingent on the ratio between macrogol 1550 (M1500) and poloxamer P338 (P338), as well as between macrogol 400 M400 and propylene glycol (PG), water content, temperature, and shear stress. It was demonstrated that P338 increases the surface-active properties of bases. The water absorption capacity of the base containing solely a mixture of macrogols is approximately 1.2 times higher than that of the base, which also contains P338 and PG. The release rate of ofloxacin is shown to increase with an increase in PG content, but it is unaffected by the replacement of Proxanol 268 with P338. The incorporation of macrogol 20 cetostearyl ether (M20CSE) and cetostearyl alcohol (CSA) markedly retards water absorption and the release of ofloxacin, and also increases the rheological parameters of the bases. It was demonstrated by the spin probe method that, within a non-aqueous medium, no aggregates are formed from molecules of P338, as well as surfactant and CSA molecules. The PG content affects the growth inhibition zones of P. aeruginosa. The antibacterial efficacy of ointments containing fluoroquinolones against resistant clinical bacterial strains was found to be enhanced by hydrophilic bases.
Conclusions. The rheological parameters of hydrophilic bases can be controlled by modifying the ratio between consistency factors and dispersion medium components, by varying water content, temperature, and shear stress, as well as by adding surfactants and CSA to their formulations. Hydrophilic bases are able to absorb water and they promote the release of ofloxacin. Surfactant and CSA have a significant impact on these processes, reducing their rates. The formation of aggregates from P338 molecules, as well as molecules of surface-active substance and CSA, was not observed in the hydrophilic bases. PG, when incorporated into hydrophilic ointments containing ofloxacin, enhances their antibacterial efficacy
Supporting Agency
- National Academy of Sciences of Ukraine within the framework of the project «Study of dispersed systems with liquid dispersion medium as the primary matrices for medicinal products» (0125U000740)
References
- Buckingham, R. (Ed.) (2020). Martindale: The Complete Drug Reference, 40th Ed. London: Pharmaceutical Press, 4852.
- Kale, V. R., Pawar, D. C., Ige, P. P. (2019). Topical preparation past, present and future perspective – a review. Indo American Journal of Pharmaceutical Sciences, 6 (5), 10094‒10110. https://doi.org/10.5281/zenodo.2978025
- The European Pharmacopoeia (2022). European Directorate for the Quality of Medicines & HealthCare of the Council of Europe. Strasbourg: Council of Europe, 6106. Available at: http://pheur.edqm.eu/subhome/11-8
- Derzhavna Farmakopeia Ukrainy. Vol. 2 (2024). Kharkiv: Derzhavne pidpryiemstvo «Ukrainskyi naukovyi farmakopeinyi tsentr yakosti likarskykh zasobiv», 424.
- Bouwman-Boer, Y., Fenton-May, V’I., Le Brun, P. (Eds.) (2009). Practical Pharmaceutics. An International Guideline for the Preparation, Care and Use of Medicinal Products. Springer International Publishing AG, 873. https://doi.org/10.1007/978-3-319-15814-3
- Sheskey, P. J., Hancock, B. C., Moss, G. P., Goldfarb, D. J. (Eds.) (2020). Handbook of Pharmaceutical Excipients. London: Pharm. Press, 1296.
- British Pharmacopoeia (2025). London: The Stationery Office. Available at: https://www.pharmacopoeia.com/
- Datsenko, B. M. (Ed.) (1995). Teoriia i praktika mestnogo lecheniia gnoinykh ran. Kyiv: Zdorove, 384.
- Derzhavnyi reiestr likarskykh zasobiv Ukrainy. Available at: http://www.drlz.kiev.ua/
- Rus, L. M., Donici, E., Valica, V., Prisacari, V., Tomuță, I., Șepeli, D. et al. (2019). Development, physical-chemical characterization and in vitro antibacterial activity evaluation of a fixed-dose combination isohydrafural-methyluracil hydrophilic ointment. Farmacia, 67 (5), 857‒865. https://doi.org/10.31925/farmacia.2019.5.15
- Dua, K., Malipeddi, V. R., Madan, J., Gupta, G., Chakravarthi, S., Awasthi, R. et al. (2016). Norfloxacin and metronidazole topical formulations for effective treatment of bacterial infections and burn wounds. Interventional Medicine and Applied Science, 8 (2), 68–76. https://doi.org/10.1556/1646.8.2016.2.4
- Noda, Y., Watanabe, K., Sanagawa, A., Sobajima, Y., Fujii, S. (2011). Physicochemical properties of macrogol ointment and emulsion ointment blend developed for regulation of water absorption. International Journal of Pharmaceutics, 419 (1-2), 131–136. https://doi.org/10.1016/j.ijpharm.2011.07.034
- Lyapunov, N., Bezuglaya, E., Liapunova, A., Zinchenko, I., Liapunov, O., Lysokobylka, O., Stolper, Y. (2022). Effect of the composition of emulsifiers and the dispersion medium on the properties of bases for semi-solid preparations. ScienceRise: Pharmaceutical Science, 5 (39), 29–45. https://doi.org/10.15587/2519-4852.2022.266001
- Murasawa, Y., Furuta, K., Noda, Y., Nakamura, H., Fujii, S., Isogai, Z. (2018). Ointment vehicles regulate the wound‐healing process by modifying the hyaluronan‐rich matrix. Wound Repair and Regeneration, 26 (6), 437–445. https://doi.org/10.1111/wrr.12673
- Otake, H., Mano, Y., Deguchi, S., Ogata, F., Kawasaki, N., Nagai, N. (2023). Effect of Ointment Base on the Skin Wound-Healing Deficits in Streptozotocin-Induced Diabetic Rat. Biological and Pharmaceutical Bulletin, 46 (5), 707–712. https://doi.org/10.1248/bpb.b22-00871
- Yamada, Y., Ueda, Y., Ashizuka, Y., Hashimoto, K., Tabara, K., Kitano, T., Fujikawa, M., Kumagai, Y., Matsumoto, T. (2018). Influence of Bases for External Medicines with Different Coatability and Water Retentivity on Wound Healing. Yakugaku Zasshi, 138 (11), 1417–1424. https://doi.org/10.1248/yakushi.18-00098
- Okusa, N., Oh, H., Masuno, K., Makita, Y., Imamura, Y. (2023). Comparative Study of Ozonated Glycerol and Macrogol Ointment on Bone Matrix Production by Human Osteosarcoma Cell Line Saos-2. Materials, 16 (10), 3857. https://doi.org/10.3390/ma16103857
- Gemeda, N., Tadele, A., Lemma, H., Girma, B., Addis, G., Tesfaye, B. et al. (2018). Development, Characterization, and Evaluation of Novel Broad‐Spectrum Antimicrobial Topical Formulations from Cymbopogon martini (Roxb.) W. Watson Essential Oil. Evidence-Based Complementary and Alternative Medicine, 2018 (1). Portico. https://doi.org/10.1155/2018/9812093
- Goo, Y. T., Kang, T. H., Lee, K. W., Kim, D. H., Park, Y. H., Kim, B. D. et al. (2020). Polyethylene Glycol-based Ointment Formulations of Alnus Sibirica Extract and Their Accelerated Stability Assessments. Polymer Korea, 44 (2), 208–218. https://doi.org/10.7317/pk.2020.44.2.208
- Nguyen, T. T. B., Nguyen, T. H., Nguyen, X. T. (2022). Preparation and Antibacterial Evaluation of Polyethylene Glycol Ointment Containing In Situ Silver Chloride Nanoparticles. BioNanoScience, 12 (1), 203–209. https://doi.org/10.1007/s12668-021-00935-1
- Swathy, B., Menaka, M., Reddy, P. (2022). Formulation and Pharmacological Screening of in vivo Wound Healing Activity of Puerarin Ointment Isolated from Tridax procumbens. Latin American Journal of Pharmacy, 41 (12), 2494‒2501.
- Lu, Y., Xiao, Y., Yin, M.-Z., Zhou, X.-C., Wu, L.-S., Chen, W.-Q. et al. (2021). Polyethylene Glycol Ointment Alleviates Psoriasis-Like Inflammation Through Down-Regulating the Function of Th17 Cells and MDSCs. Frontiers in Medicine, 7. https://doi.org/10.3389/fmed.2020.560579
- Shmatenko, O., Kazmirchuk, A., Solomennyy, A., Syrota, P., Plieshkova, O., Davtian, L. (2021). Rationale for Choosing the Basis for Early Coverage. Archives Of Pharmacy Practice, 12 (1), 103–108. https://doi.org/10.51847/g1cluwbev3
- Bhagurkar, A. M., Angamuthu, M., Patil, H., Tiwari, R. V., Maurya, A., Hashemnejad, S. M. et al. (2015). Development of an Ointment Formulation Using Hot-Melt Extrusion Technology. AAPS PharmSciTech, 17 (1), 158–166. https://doi.org/10.1208/s12249-015-0453-3
- Bodratti, A., Alexandridis, P. (2018). Formulation of Poloxamers for Drug Delivery. Journal of Functional Biomaterials, 9 (1), 11. https://doi.org/10.3390/jfb9010011
- Bodratti, A. M., Sarkar, B., Alexandridis, P. (2017). Adsorption of poly(ethylene oxide)-containing amphiphilic polymers on solid-liquid interfaces: Fundamentals and applications. Advances in Colloid and Interface Science, 244, 132–163. https://doi.org/10.1016/j.cis.2016.09.003
- Liapunov, N. A., Bezuhlaia, E. P., Fadeikyna, A. H., Lisokobilka, A. A., Stolper, Yu. M. (1999). Sozdanye miahkykh lekarstvennikh sredstv na razlychnikh osnovakh. Soobshchenye 1. Yssledovanye reolohycheskykh svoistv mazei na vodorastvorymikh osnovakh. Farmakom, 6, 10–16.
- Bezuglaya, E., Krasnopyorova, A., Liapunova, A., Zinchenko, I., Lyapunov, N., Sytnik, O. (2023). Influence of physicochemical properties and structure of mixed solvents propylene glycol – macrogol 400 on their in vitro release. ScienceRise: Pharmaceutical Science, 1 (41), 4–13. https://doi.org/10.15587/2519-4852.2023.274468
- Bezugla, O. P., Lyapunov, M. O., Zinchenko, I. O., Lisokobilka, O. A., Liapunova, A. M. (2022). Modeling of processes of solvent diffusion from ointment bases using in vitro experiments. Functional materials, 29 (4), 553–558. https://doi.org/10.15407/fm29.04.553
- Ilić, T., Pantelić, I., Savić, S. (2021). The Implications of Regulatory Framework for Topical Semisolid Drug Products: From Critical Quality and Performance Attributes towards Establishing Bioequivalence. Pharmaceutics, 13 (5), 710. https://doi.org/10.3390/pharmaceutics13050710
- Tiffner, K. I., Kanfer, I., Augustin, T., Raml, R., Raney, S. G., Sinner, F. (2018). A comprehensive approach to qualify and validate the essential parameters of an in vitro release test (IVRT) method for acyclovir cream, 5%. International Journal of Pharmaceutics, 535 (1-2), 217–227. https://doi.org/10.1016/j.ijpharm.2017.09.049
- The United States Pharmacopoeia 47 ed. The National Formulary 42 [USP 47 – NF 42] (2023). The United States Pharmacopoeial Convention. Rockville: United Book Press, Inc.
- Berliner, L. J., Reuben, J. (Ed.) (1989). Spin Labeling: Theory and Applications. New York: Plenum Press, 670. https://doi.org/10.1007/978-1-4613-0743-3
- Balouiri, M., Sadiki, M., Ibnsouda, S. K. (2016). Methods for in vitro evaluating antimicrobial activity: A review. Journal of Pharmaceutical Analysis, 6 (2), 71–79. https://doi.org/10.1016/j.jpha.2015.11.005
- Lipson, V., Bezugla, O., Dzhoraieva, S., Kutasevych, Y., Liapunova, A., Zinchenko, I. et al. (2025). Effect of 3,3′-Diindolylmethane on the Antibacterial Activity of Fluoroquinolones as Constituents of Potential Drug Products for Topical Application. ACS Applied Materials & Interfaces, 17 (34), 47906–47918. https://doi.org/10.1021/acsami.5c08154
- Lapach, S. M., Chubenko, A. V., Babich, P. N. (2002). Statystyka v nautsi ta biznesi. Kyiv: MORION, 640.
- Lyapunov, N., Bezuglaya, E., Liapunov, O., Lysokobylka, O. (2023). Study of aqueous solutions of poloxamers by rotational viscometry and spin probe method. ScienceRise: Pharmaceutical Science, 4 (44), 4–18. https://doi.org/10.15587/2519-4852.2023.285933
- Liapunova, A. M., Krasnopyorova, А. P., Bezuglа, О. P., Liapunov, O. M., Yukhnо, G. D., Pukhova, T. М. (2024). Polythermal studies of the water – propylene glycol systems by densitometry, viscometry and spin probes method. Functional Materials, 31 (4), 609–618. https://doi.org/10.15407/fm31.04.609
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Nikolay Lyapunov, Оlena Bezugla, Anna Liapunova, Igor Zinchenko, Oleksii Liapunov, Oleksii Lysokobylka, Svitlana Dzhoraіeva

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






