Investigating the potential effect of l-citrulline on PI3K signaling pathway: in silico and in vitro evaluation
DOI:
https://doi.org/10.15587/2519-4852.2025.342290Keywords:
Insulin resistance, Type 2 diabetes mellitus, L-Citrulline, Metformin, PI3K signaling pathway, ADME, In vitro, In silico, Molecular docking, Skeletal muscle cellsAbstract
Insulin resistance is a key feature of type 2 diabetes mellitus (T2DM), resulting from dysfunction in the insulin signaling pathway, which involves critical proteins such as IRS-PI3K-IRS-1-PKC-AKT2 and GLUT4. Metformin, a first-line T2DM treatment, exerts its effects via various mechanisms, but alternative therapies are needed. L-Citrulline, an amino acid with antiglycation and antioxidant properties, has shown potential as a therapeutic agent.
The aim. This study aims to evaluate the efficacy of L-Citrulline, alongside the well-established antidiabetic drug metformin, in a T2DM model using both in vitro and in silico approaches.
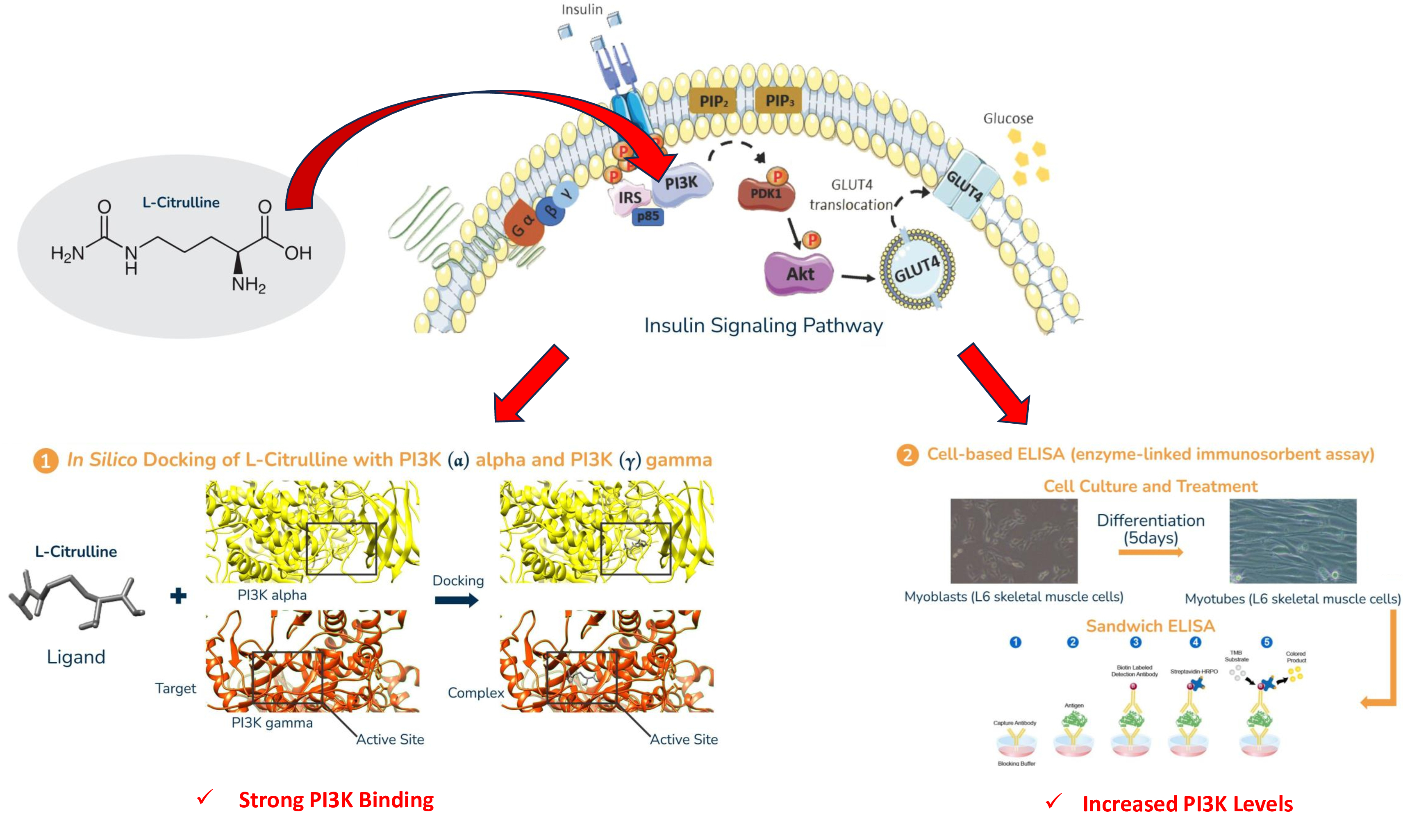
Materials and methods. Differentiated L6 skeletal muscle cells, induced with high glucose and insulin concentrations to model insulin resistance, were treated with either L-Citrulline or metformin. The expression of PI3K, a key protein in insulin signaling, was assessed using an ELISA Kit. In silico molecular docking studies were also conducted to examine the binding interactions of L-Citrulline and metformin with PI3K.
Results. L-Citrulline treatment significantly increased PI3K concentration levels in insulin-resistant skeletal muscle cells, indicating a potential restoration of insulin signaling. The enhancement in PI3K concentration was comparable to that observed with metformin, validating the effectiveness of L-Citrulline in modulating the PI3K pathway. Molecular docking studies revealed that L-Citrulline formed stable and favorable interactions with PI3K, suggesting strong binding affinity and potential enhancement of its catalytic activity.
Conclusion. L-Citrulline demonstrates potential in modulating the PI3K signaling pathway based on both in vitro and in silico findings, indicating a possible role in improving insulin responsiveness in type 2 diabetes mellitus (T2DM). Nevertheless, these results are preliminary, and further in vivo and clinical investigations are needed to confirm its therapeutic relevance
Supporting Agency
- DOST-ASTHRDP and the DOST- Research Enrichment Program for the purpose of conducting this research
References
- Galicia-Garcia, U., Benito-Vicente, A., Jebari, S., Larrea-Sebal, A., Siddiqi, H., Uribe, K. B. et al. (2020). Pathophysiology of Type 2 Diabetes Mellitus. International Journal of Molecular Sciences, 21 (17), 6275. https://doi.org/10.3390/ijms21176275
- Olokoba, A. B., Obateru, O. A., Olokoba, L. B. (2012). Type 2 Diabetes Mellitus: A Review of Current Trends. Oman Medical Journal, 27 (4), 269–273. https://doi.org/10.5001/omj.2012.68
- Saeedi, P., Petersohn, I., Salpea, P., Malanda, B., Karuranga, S., Unwin, N. et al. (2019). Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Research and Clinical Practice, 157, 107843. https://doi.org/10.1016/j.diabres.2019.107843
- Saltiel, A. R., Kahn, C. R. (2001). Insulin signalling and the regulation of glucose and lipid metabolism. Nature, 414 (6865), 799–806. https://doi.org/10.1038/414799a
- Nwabueze, O. P., Sharma, M., Balachandran, A., Gaurav, A., Abdul Rani, A. N., Małgorzata, J. et al. (2022). Comparative Studies of Palmatine with Metformin and Glimepiride on the Modulation of Insulin Dependent Signaling Pathway In Vitro, In Vivo & Ex Vivo. Pharmaceuticals, 15 (11), 1317. https://doi.org/10.3390/ph15111317
- Huang, X., Liu, G., Guo, J., Su, Z. (2018). The PI3K/AKT pathway in obesity and type 2 diabetes. International Journal of Biological Sciences, 14 (11), 1483–1496. https://doi.org/10.7150/ijbs.27173
- L-citrulline supplementation for improving glycemic control and markers of inflammation in type 2 diabetes. Examine. Available at: https://examine.com/research-feed/study/dVgWP1/ Last accessed: 10.04.2024
- Romero, M. J., Platt, D. H., Caldwell, R. B., Caldwell, R. W. (2006). Therapeutic Use of Citrulline in Cardiovascular Disease. Cardiovascular Drug Reviews, 24 (3-4), 275–290. https://doi.org/10.1111/j.1527-3466.2006.00275.x
- Flores-Ramírez, A. G., Tovar-Villegas, V. I., Maharaj, A., Garay-Sevilla, M. E., Figueroa, A. (2021). Effects of L-Citrulline Supplementation and Aerobic Training on Vascular Function in Individuals with Obesity across the Lifespan. Nutrients, 13 (9), 2991. https://doi.org/10.3390/nu13092991
- Paulines, J. M. U., Lavilla Jr, C. A., Billacura, M. P., Basalo, H. L., Okechukwu, P. N. (2023). In vitro evaluation of the antiglycation and antioxidant potential of the dietary supplement L-citrulline. ScienceRise: Pharmaceutical Science, 4 (44), 46–53. https://doi.org/10.15587/2519-4852.2023.286542
- Hooper, C. (2019). An overview of insulin signaling pathways. Abcam. Available at: https://www.abcam.com/pathways/overview-of-insulin-signaling-pathways
- Zhang, Z.-Y., Miao, L.-F., Qian, L.-L., Wang, N., Qi, M.-M., Zhang, Y.-M. et al. (2019). Molecular Mechanisms of Glucose Fluctuations on Diabetic Complications. Frontiers in Endocrinology, 10. https://doi.org/10.3389/fendo.2019.00640
- Lavilla, C., Turner, M. (2020). Carnosine in skeletal muscle: biological action and therapeutic implications. Available at: https://irep.ntu.ac.uk/id/eprint/42070/1/CHARLIE.LAVILLA%20JR%202020%20excl3rdpartycopyright.pdf
- AL-Ishaq, R. K., Abotaleb, M., Kubatka, P., Kajo, K., Büsselberg, D. (2019). Flavonoids and Their Anti-Diabetic Effects: Cellular Mechanisms and Effects to Improve Blood Sugar Levels. Biomolecules, 9 (9), 430. https://doi.org/10.3390/biom9090430
- Allerton, T., Proctor, D., Stephens, J., Dugas, T., Spielmann, G., Irving, B. (2018). l-Citrulline Supplementation: Impact on Cardiometabolic Health. Nutrients, 10 (7), 921. https://doi.org/10.3390/nu10070921
- Kurhaluk, N., Tkaczenko, H. (2025). L-Arginine and Nitric Oxide in Vascular Regulation – Experimental Findings in the Context of Blood Donation. Nutrients, 17 (4), 665. https://doi.org/10.3390/nu17040665
- Quade-Lyssy, P., Kanarek, A. M., Baiersdörfer, M., Postina, R., Kojro, E. (2013). Statins stimulate the production of a soluble form of the receptor for advanced glycation end products. Journal of Lipid Research, 54 (11), 3052–3061. https://doi.org/10.1194/jlr.m038968
- Čater, M., Hölter, S. M. (2022). A Pathophysiological Intersection of Diabetes and Alzheimer’s Disease. International Journal of Molecular Sciences, 23 (19), 11562. https://doi.org/10.3390/ijms231911562
- Fotheringham, A. K., Gallo, L. A., Borg, D. J., Forbes, J. M. (2022). Advanced Glycation End Products (AGEs) and Chronic Kidney Disease: Does the Modern Diet AGE the Kidney? Nutrients, 14 (13), 2675. https://doi.org/10.3390/nu14132675
- Nowotny, K., Jung, T., Höhn, A., Weber, D., Grune, T. (2015). Advanced Glycation End Products and Oxidative Stress in Type 2 Diabetes Mellitus. Biomolecules, 5 (1), 194–222. https://doi.org/10.3390/biom5010194
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Jessa Marielle U. Paulines, Patrick Nwabueze Okechukwu, Charlie A. Lavilla Jr

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






