Застосування арбутину для знаття резистентності антибіотиків проти грамнегативних бактерій із множинною лікарською стійкістю Pseudomonas aeruginosa та Enterobacter cloacea
DOI:
https://doi.org/10.15587/2519-4852.2024.305965Ключові слова:
арбутин, мультирезистентність, грамнегативні штами, молекулярний докінг, знаття резистентності, антибіотикиАнотація
Основним методом терапії проти мікробних інфекцій є застосування антибіотиків. Однак, надмірне використання антибіотиків стало основним фактором виникнення і поширення мультирезистентних штамів.
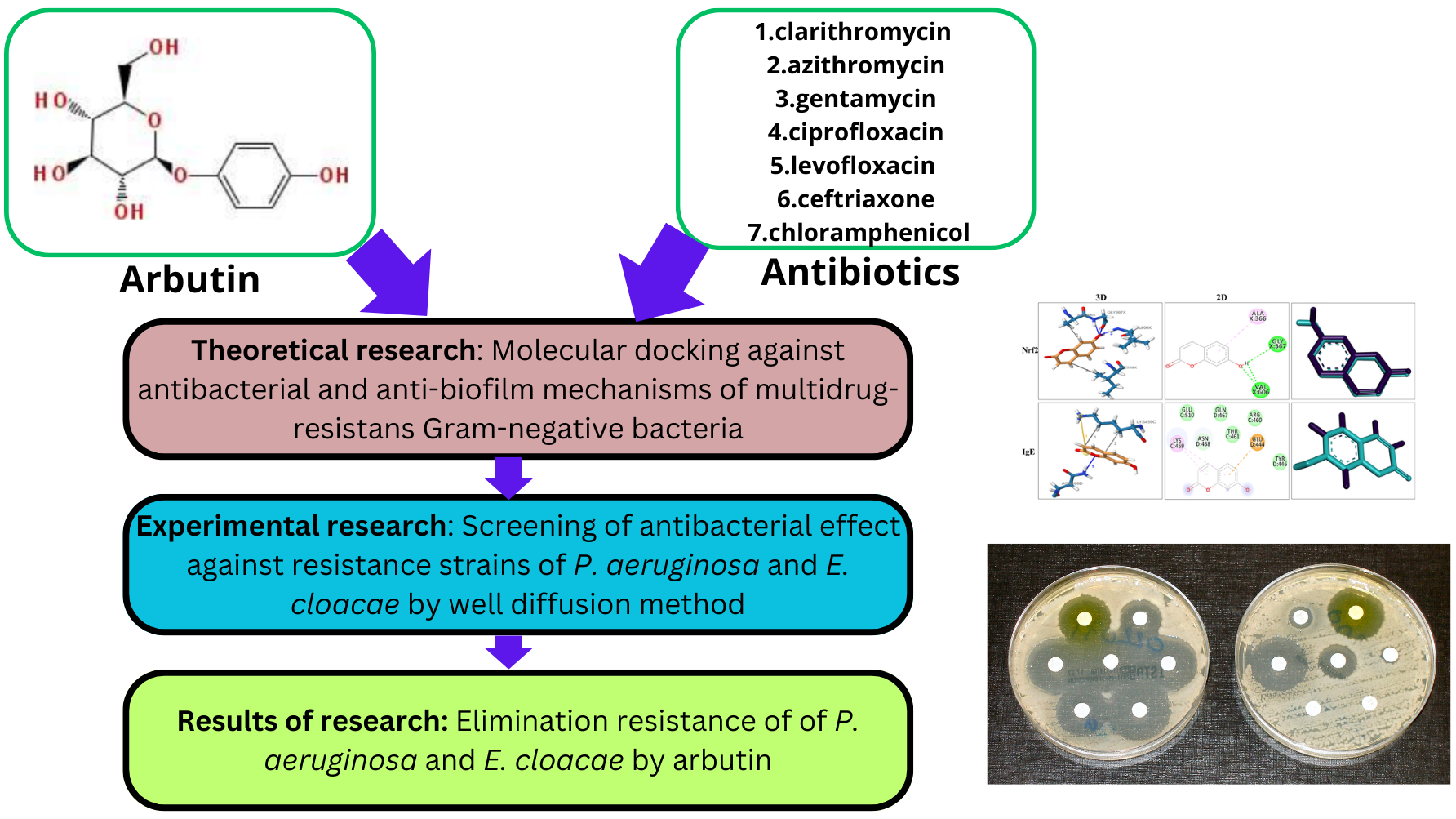
Мета. Метою нашої роботи було дослідити in vitro та in silico елімінацію резистентності до антибіотиків (кларитроміцин, азитроміцин, гентаміцин, ципрофлоксацин, левофлоксацин, цефтріаксон і хлорамфенікол) проти клінічних мультирезистентних штамів P. aeruginosa, E. cloacae за допомогою арбутину. Всі грамнегативні резистентні штами бактерій були чутливими до дії арбутину.
Матеріали та методи. Молекулярне докінгування виконувалося за допомогою AutoDockTools 1.5.6; антимікробні ефекти оцінювалися методом лунок. Ізоляти були отримані з клінічних зразків, включаючи аспірат трахеї та бронкоальвеолярний лаваж.
Результати. Теоретичні дослідження показали, що жоден із досліджених антибіотиків та арбутину не інгібує високоселективно всі "цілі" механізмів антимікробної дії. В експериментальних дослідженнях спостерігалося, що додавання арбутину до антибіотика призводило до появи чутливості з боку резистентних штамів. Крім того, арбутин збільшував антимікробний ефект антибіотиків від 8 до 55 %. Виключенням стали кларитроміцин та азитроміцин при оцінці антимікробної активності проти P. aeruginosa.
Висновки. Ці дослідження показали, що для інгібування резистентних штамів бактерій необхідно використовувати комбінації "класичних" антимікробних засобів та рослинних препаратів або дієтичних добавок на основі екстрактів, отриманих з арбутинвмісних лікарських рослин, таких як брусниця, мучниця і журавлина. Такий підхід є "рятувальним колом" для розробки антимікробних засобів проти резистентних бактерій і дає "другий шанс на життя" для застарілих антибіотиків
Спонсор дослідження
- Ministry of Health of Ukraine, carried out at the expense of the state budget of Ukraine No. 0124U002080 (Order of the Ministry of Health of Ukraine No. 17 of 27.02.2024). We are grateful for the scientific and material help provided by the pharmaceutical company "Astrapharm" Kyiv, Ukraine, and the pharmaceutical company "Zdravopharm", Kharkiv, Ukraine.
Посилання
- Mende, K., Akers, K. S., Tyner, S. D., Bennett, J. W., Simons, M. P., Blyth, D. M. et al. (2022). Multidrug-Resistant and Virulent Organisms Trauma Infections: Trauma Infectious Disease Outcomes Study Initiative. Military Medicine, 187 (Supplement_2), 42–51. https://doi.org/10.1093/milmed/usab131
- Kondratiuk, V., Jones, B. T., Kovalchuk, V., Kovalenko, I., Ganiuk, V., Kondratiuk, O., Frantsishko, A. (2021). Phenotypic and genotypic characterization of antibiotic resistance in military hospital-associated bacteria from war injuries in the Eastern Ukraine conflict between 2014 and 2020. Journal of Hospital Infection, 112, 69–76. https://doi.org/10.1016/j.jhin.2021.03.020
- Petrosillo, N., Petersen, E., Antoniak, S. (2023). Ukraine war and antimicrobial resistance. The Lancet Infectious Diseases, 23 (6), 653–654. https://doi.org/10.1016/s1473-3099(23)00264-5
- Antimicrobial resistance surveillance in Europe 2022. 2020 data (2022). WHO Regional Office for Europe (WHO/Europe)/European Centre for Disease Prevention and Control (ECDC). Copenhagen: WHO/Europe. Available at: https://www.ecdc.europa.eu/sites/default/files/documents/ECDC-WHO-AMR-report.pdf
- Schultze, T., Hogardt, M., Velázquez, E. S., Hack, D., Besier, S., Wichelhaus, T. A. et al. (2023). Molecular surveillance of multidrug-resistant Gram-negative bacteria in Ukrainian patients, Germany, March to June 2022. Eurosurveillance, 28 (1). https://doi.org/10.2807/1560-7917.es.2023.28.1.2200850
- Maslov, O., Komisarenko, M., Kolisnyk, S., Tkachenko, O., Akhmedov, E., Poluain, S. et al. (2023). Study of qualitative composition and quantitative content of free organic acids in lingberry leaves. Fitoterapia, 1, 77–82. https://doi.org/10.32782/2522-9680-2023-1-77
- Zhou, H., Zhao, J., Li, A., Reetz, M. T. (2019). Chemical and Biocatalytic Routes to Arbutin †. Molecules, 24 (18), 3303. https://doi.org/10.3390/molecules24183303
- Ma, C., He, N., Zhao, Y., Xia, D., Wei, J., Kang, W. (2019). Antimicrobial Mechanism of Hydroquinone. Applied Biochemistry and Biotechnology, 189 (4), 1291–1303. https://doi.org/10.1007/s12010-019-03067-1
- Maslov, O., Komisarenko, M., Ponomarenko, S., Horopashna, D., Osolodchenko, T., Kolisnyk, S. et al. (2022). Investigation the influence of biologically active compounds on the antioxidant, antibacterial and anti-inflammatory activities of red raspberry (Rubus idaeous l.) leaf extract. Current Issues in Pharmacy and Medical Sciences, 35 (4), 229–235. https://doi.org/10.2478/cipms-2022-0040
- Osolodchenko, T., Andreieva, I., Martynov, A., Nadiya, Z., Batrak, O., Ryabova, I. (2024). Monitoring of time stability of antimicrobial effect of nisin-based pharmaceutical compositions with resistance inhibitors. Annals of Mechnikov Institute, 3, 40–46. https://doi.org/10.5281/zenodo.13819951
- Morris, G. M., Huey, R., Olson, A. J. (2008). Using AutoDock for Ligand‐Receptor Docking. Current Protocols in Bioinformatics, 24 (1). https://doi.org/10.1002/0471250953.bi0814s24
- RCSB PDB: Homepage. RCSB PDB: Homepage. Available at: https://www.rcsb.org/
- PubChem. Available at: https://pubchem.ncbi.nlm.nih.gov/
- CASTp 3.0: Computed Atlas of Surface Topography of proteins. Available at: http://sts.bioe.uic.edu/castp/index.html?201l
- Kondža, M., Brizić, I., Jokić, S. (2024). Flavonoids as CYP3A4 Inhibitors In Vitro. Biomedicines, 12 (3), 644. https://doi.org/10.3390/biomedicines12030644
- Pulingam, T., Parumasivam, T., Gazzali, A. M., Sulaiman, A. M., Chee, J. Y., Lakshmanan, M. et al. (2022). Antimicrobial resistance: Prevalence, economic burden, mechanisms of resistance and strategies to overcome. European Journal of Pharmaceutical Sciences, 170, 106103. https://doi.org/10.1016/j.ejps.2021.106103
- Abinaya, M., Gayathri, M. (2019). Inhibition of biofilm formation, quorum sensing activity and molecular docking study of isolated 3, 5, 7-Trihydroxyflavone from Alstonia scholaris leaf against P.aeruginosa. Bioorganic Chemistry, 87, 291–301. https://doi.org/10.1016/j.bioorg.2019.03.050
- Jogula, S., Krishna, V. S., Meda, N., Balraju, V., Sriram, D. (2020). Design, synthesis and biological evaluation of novel Pseudomonas aeruginosa DNA gyrase B inhibitors. Bioorganic Chemistry, 100, 103905. https://doi.org/10.1016/j.bioorg.2020.103905
- Mbarga, M. J. A., Podoprigora, I. V., Volina, E. G., Ermolaev, A. V., Smolyakova, L. A. (2021). Evaluation of Changes Induced in the Probiotic Escherichia coli M17 Following Recurrent Exposure to Antimicrobials. Journal of Pharmaceutical Research International, 158–167. https://doi.org/10.9734/jpri/2021/v33i29b31601
- Zuo, K., Liang, L., Du, W., Sun, X., Liu, W., Gou, X. et al. (2017). 3D-QSAR, Molecular Docking and Molecular Dynamics Simulation of Pseudomonas aeruginosa LpxC Inhibitors. International Journal of Molecular Sciences, 18 (5), 761. https://doi.org/10.3390/ijms18050761
- Abinaya, M., Gayathri, M. (2019). Inhibition of biofilm formation, quorum sensing activity and molecular docking study of isolated 3, 5, 7-Trihydroxyflavone from Alstonia scholaris leaf against P.aeruginosa. Bioorganic Chemistry, 87, 291–301. https://doi.org/10.1016/j.bioorg.2019.03.050
- Jean, S.-S., Harnod, D., Hsueh, P.-R. (2022). Global Threat of Carbapenem-Resistant Gram-Negative Bacteria. Frontiers in Cellular and Infection Microbiology, 12. https://doi.org/10.3389/fcimb.2022.823684
- Aranaga, C., Pantoja, L. D., Martínez, E. A., Falco, A. (2022). Phage Therapy in the Era of Multidrug Resistance in Bacteria: A Systematic Review. International Journal of Molecular Sciences, 23 (9), 4577. https://doi.org/10.3390/ijms23094577
##submission.downloads##
Опубліковано
Як цитувати
Номер
Розділ
Ліцензія
Авторське право (c) 2024 Oleksandr Maslov, Mykola Komisarenko , Svitlana Ponomarenko, Tetiana Osolodchenko, Sergii Kolisnyk , Oleh Koshovyi, Andrey Komissarenko

Ця робота ліцензується відповідно до Creative Commons Attribution 4.0 International License.
Наше видання використовує положення про авторські права Creative Commons CC BY для журналів відкритого доступу.







