Investigation of the profile of dry extracts of Iris hungarica leaves and rhizome to determine the cardioprotective activity in the rat model of doxorubicin cardiomyopathy
DOI:
https://doi.org/10.15587/2519-4852.2024.310779Keywords:
doxorubicin cardiomyopathy, cardioprotective activity, potassium orotate, Iris hungarica leaves dry extract, Iris hungarica rhizomes dry extract, phenolic compounds, HPLCAbstract
The search and creation of new cardioprotective drugs, especially of plant origin, with a prolonged effect and a minimum of side effects, is an urgent task to improve the prognosis of cardiovascular diseases, prevent the risk of developing complications, and increase the duration and quality of life of patients. Iris hungarica, from the Iridaceae family, has a long history of medicinal use in many countries of the world and is also recognized as a rich source of BAC.
The aim. Study of the cardioprotective effect of dry extracts of leaves and rhizomes of Iris hungarica on the rat model of doxorubicin cardiomyopathy.
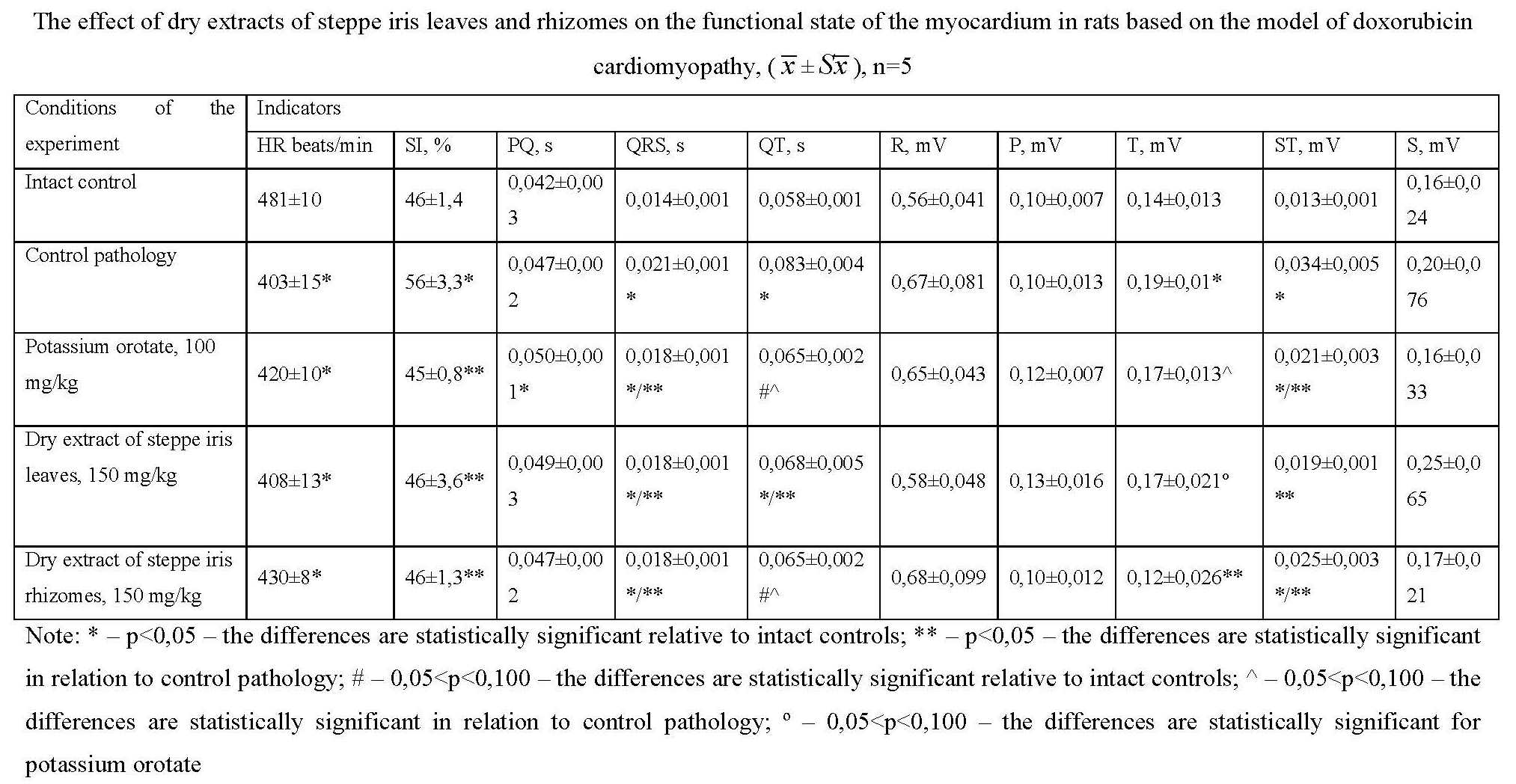
Materials and methods. The research was conducted on 40 white outbred female rats, which were injected intraperitoneally with a solution of doxorubicin hydrochloride at a dose of 1 mg/kg, at the rate of 0.5 mL per 100 g of the animal's body weight, according to the scheme 2 times a week for 6 weeks. The cardiotoxic effect and protective properties of potassium orotate, dry extracts of leaves and rhizomes of steppe iris were evaluated by animal survival, determination of the relative heart mass ratio, functional state of the myocardium (ECG parameters) and biochemical parameters in blood serum and heart homogenate.
Results and discussion. The analysis of indicators of the functional state of the conducting system of the heart shows that the 15-day use in the treatment of animals with doxorubicin cardiomyopathy of the dry extract of the leaves and rhizomes of Iris hungarica at a dose of 150 mg/kg demonstrates a cardioprotective effect at the initial stage. In the model of doxorubicin cardiomyopathy, the dry extracts of the leaves and rhizomes of steppe iris at a dose of 150 mg/kg showed a normalizing effect on biochemical parameters in blood serum and in heart homogenate and were not inferior to the action of the comparison drug potassium orotate at a dose of 100 mg/kg.
The dry extract of the rhizomes of steppe iris revealed the most pronounced effect on metabolism in cardiomyocytes. The cardioprotective activity of the dry extract of the rhizomes of the Iris hungarica is defined as a cardioprotector - of the anabolic and antioxidant type - those that accelerate the recovery of the heart muscle, protect the heart muscle from the action of free radicals, preventing premature aging and wear.
Conclusions. In the model of doxorubicin cardiomyopathy in rats, indicators of the functional state of the conducting system of the heart of animals after the use in the treatment of animals of the dry extracts of the leaves and rhizomes of steppe iris in a dose of 150 mg/kg demonstrate a cardioprotective effect at the initial stage, a normalizing effect on biochemical indicators in the blood serum and in the homogenate heart and are not inferior to the comparison drug potassium orotate in a dose of 100 mg/kg.
The most pronounced effect of the dry extract of the rhizomes of Iris hungarica on the functional state of the myocardium and biochemical indicators in blood serum and heart homogenate was established.
The dry extract of the rhizomes of steppe iris is a promising herbal remedy for the creation of a new drug with cardioprotective properties
Supporting Agency
- National Pharmaceutical University, approved by the Ministry of Health of Ukraine: "Pharmacological study of biologically active substances and medicinal products" (state registration number 0114U000956).
References
- Nishida, K., Otsu, K. (2017). Inflammation and metabolic cardiomyopathy. Cardiovascular Research, 113(4), 389–398. https://doi.org/10.1093/cvr/cvx012
- Pelykh, V. Y., Saturska, H. S., Usynskyi, R. S. (2020). Remodeling of rat’s heart in conditions of metabolic cardiomyopathy development and possibilities of its correction. Achievements of Clinical and Experimental Medicine, 2, 140–144. https://doi.org/10.11603/1811-2471.2020.v.i2.11331
- Kerymova, H. F., Korol, V. V., Rybak, V. A. (2019). Osoblyvosti mekhanizmu dii ta zastosuvannia fitopreparativ-anabolikiv z metoiu stvorennia likarskykh preparativ na osnovi sukhoho ekstraktu Iris hungarica. Perspectives of world science and education. Osaka, 50–56.
- Strutynskyi, R. B., Rovenets, R. A., Moibenko, O. O. (2012). Mekhanizmy kardioprotektornoi dii vitchyznianoho aktyvatora KAT+ kanaliv flokalinu. Tavriiskyi medyko-biolohichnyi visnyk, 15 (3 (42 (59))), 226–229.
- Dzhyhaliuk, O. V., Stepaniuk, H. I., Zaichko, N. V., Kovalenko, S. I., Shabelnyk, K. P. (2017). Characteristics of influence of 4-[4-oxo-3 h-quinazoline-3-yl] benzoic acid (pc-66) on a course of adrenaline myocardiodystrophy in rats according to biochemical research. Medical and Clinical Chemistry, 18 (4), 16–11. https://doi.org/10.11603/mcch.2410-681x.2016.v0.i4.7249
- Witard, O. C., Combet, E., Gray, S. R. (2019). Long-chainn-3 fatty acids as an essential link between musculoskeletal and cardio-metabolic health in older adults. Proceedings of the Nutrition Society, 79 (1), 47–55. https://doi.org/10.1017/s0029665119000922
- Singab, A. N. B., Ayoub, I. M., El-Shazly, M., Korinek, M., Wu, T.-Y., Cheng, Y.-B. et al. (2016). Shedding the light on Iridaceae: Ethnobotany, phytochemistry and biological activity. Industrial Crops and Products, 92, 308–335. https://doi.org/10.1016/j.indcrop.2016.07.040
- Khatib, S., Faraloni, C., Bouissane, L. (2022). Exploring the Use of Iris Species: Antioxidant Properties, Phytochemistry, Medicinal and Industrial Applications. Antioxidants, 11 (3), 526. https://doi.org/10.3390/antiox11030526
- Syahputra, R. A., Harahap, U., Dalimunthe, A., Nasution, M. P., Satria, D. (2022). The Role of Flavonoids as a Cardioprotective Strategy against Doxorubicin-Induced Cardiotoxicity: A Review. Molecules, 27 (4), 1320. https://doi.org/10.3390/molecules27041320
- Mykhailenko, O., Korinek, M., Ivanauskas, L., Bezruk, I., Myhal, A., Petrikaitė, V. et al. (2020). Qualitative and Quantitative Analysis of Ukrainian Iris Species: A Fresh Look on Their Antioxidant Content and Biological Activities. Molecules, 25 (19), 4588–4612. https://doi.org/10.3390/molecules25194588
- Trofimova, T. S., Chekman, I. S., Horchakova, N. O., Avramenko, M. O. (2004). Kardiotoksychnist doksorubitsynu ta shliakhy yii korektsii tiotriazolinom. Zaporizkyi medychnyi zhurnal, 5, 135–155.
- Nakahara, T., Tanimoto, T., Petrov, A. D., Ishikawa, K., Strauss, H. W., Narula, J. (2018). Rat Model of Cardiotoxic Drug-Induced Cardiomyopathy. Experimental Models of Cardiovascular Diseases, 221–232. https://doi.org/10.1007/978-1-4939-8597-5_17
- Baklanova, Ya. V., Ushakova, H. O. (2013). Toksychni efekty ta biokhimichnyi kontrol naslidkiv antratsyklinovoi terapii. Arkhiv klinichnoi ta eksperymentalnoi medytsyny, 22 (1), 1–8.
- Stefanov, O. V. (Ed.) (2001). Doklinichni doslidzhennia likarskykh zasobiv. Kyiv: Avitsenna, 528.
- Derzhavna farmakopeia Ukrainy (2004). Kharkiv: Derzhavne pidpryiemstvo «Ukrainskyi naukovyi farmakopeinyi tsentr yakosti likarskykh zasobiv», 520.
- Krechun, A. V., Kierimova, H. F., Kovalov, V. M., Rybak, V. A., Mykhailenko, O. O. (2021). Pat. No. 124650 UA. Sposib oderzhannia kompleksu biolohichno aktyvnykh rechovyn z anabolichnoiu ta protyzapalnoiu aktyvnistiu z korenevyshch irysa uhorskoho. MPK: A61K 36/88 (2006.01), A61K 125/00, A61P 3/00, A61P 21/06 (2006.01), A61P 29/00. No. u201911756. declareted: 09.12.2019; published: 21.10.2021., Bul. No. 42.
- Trofimova, T. S. (2008). Eksperymentalni doslidzhennia efektyvnosti tiotryozolinu za umov doksorubitsynovoi kardiomiopatii. [PhD theses].
- Skybchyk, V. A., Skybchyk, Ya. V. (2021). Klinichna elektrokardiohrafiia. Lviv: Vydavets Marchenko T. V., 568.
- Gubskyi, Yu. I. (2020). Biological chemistry. Vinnitsa: Nova Knyha, 488.
- Lunova, H. H., Lipkan, H. M., Viunytska, L. V. et al.; Lunova, H. H. (Ed.) (2022). Klinichna biokhimiia. Vol. 3. Lviv: PP «Mahnoliia 2006», 296.
- Chen, Z., Chen, L., Dai, H., Chen, J., Fang, L. (2008). Relationship between alanine aminotransferase levels and metabolic syndrome in nonalcoholic fatty liver disease. Journal of Zhejiang University SCIENCE B, 9 (8), 616–622. https://doi.org/10.1631/jzus.b0720016
- Stalnaia, I. D., Garishvili, G. T.; Orekhovicha, V. A. (Ed.) (1977). Metod opredeleniia malonovogo dialdegida s pomoshchiu tiobarbiturovoi kisloty. Sovremennye metody biokhimii. Moscow: Meditcina, 43–44.
- Beutler, Е., Duron, O., Kelly, B. M. (1963). Improved method for the determination of blood glutathion. Journal of Laboratory and Clinical Medicine, 63 (5), 882–888.
- Koroliuk, M. A. Ivanova, L. I., Maiorova, I. G., Tokarev, V. E. (1988). Metod opredeleniia aktivnosti katalazy. Laboratornoe delo, 1, 16–19.
- Lapach, S. N., Chubenko, A. V., Babich, P. N. (2000). Statisticheskie metody v mediko-biologicheskikh issledovaniiakh s ispolzovaniem Excel. Kyiv: Morion, 320.
- Osnovnye metody statisticheskoi obrabotki rezultatov farmakologicheskikh eksperimentov (2000). Rukovodstvo po eksperimentalnomu (doklinicheskomu) izucheniiu novykh farmakologicheskikh veshchestv. Moscow: Remedium, 349–354.
- Korniievskyi, Yu. I., Kraidashenko, O. V., Krasko, M. P., Bohuslavska, N. Yu., Korniievska, V. H. (2017). Fitoterapiia v kardiolohii. Zaporizhzhia: Vyd-vo ZDMU, 470.
- Qu, D., Han, J., Ren, H., Yang, W., Zhang, X., Zheng, Q., Wang, D. (2015). Cardioprotective Effects of Astragalin against Myocardial Ischemia/Reperfusion Injury in Isolated Rat Heart. Oxidative Medicine and Cellular Longevity, 2016 (1). https://doi.org/10.1155/2016/8194690
- Mykhailenko, O., Ivanauskas, L., Bezruk, I., Lesyk, R., Georgiyants, V. (2020). Comparative Investigation of Amino Acids Content in the Dry Extracts of Juno bucharica, Gladiolus Hybrid Zefir, Iris Hungarica, Iris Variegata and Crocus Sativus Raw Materials of Ukrainian Flora. Scientia Pharmaceutica, 88 (1), 8–21. https://doi.org/10.3390/scipharm88010008
- Gueta, I., Perach Ovadia, Y., Markovits, N., Schacham, Y. N., Epsztein, A., Loebstein, R. (2020). Is Pyroglutamic Acid a Prognostic Factor Among Patients with Suspected Infection? A Prospective Cohort Study. Scientific Reports, 10 (1). https://doi.org/10.1038/s41598-020-66941-7
- Archakova, L. I., Novakovskaia, S. A. (2017). Kletochnye mekhanizmy antratciklinovoi kardiomiopatii pri deistvii antibiotika doksorubitcina. Vestcі Natcyianalnoi akademіі navuk Belarusі. Seryia medytcynskіkh navuk, 1, 83–89.
- Octavia, Y., Tocchetti, C. G., Gabrielson, K. L., Janssens, S., Crijns, H. J., Moens, A. L. (2012). Doxorubicin-induced cardiomyopathy: From molecular mechanisms to therapeutic strategies. Journal of Molecular and Cellular Cardiology, 52 (6), 1213–1225. https://doi.org/10.1016/j.yjmcc.2012.03.006
- Rahimi_Balaei, M., Momeny, M., Babaeikelishomi, R., Ejtemaei Mehr, S., Tavangar, S. M., Dehpour, A. R. (2010). The modulatory effect of lithium on doxorubicin-induced cardiotoxicity in rat. European Journal of Pharmacology, 641 (2-3), 193–198. https://doi.org/10.1016/j.ejphar.2010.05.046
- Mykhailenko, O., Hsieh, C.-F., El-Shazly, M., Nikishin, A., Kovalyov, V., Shynkarenko, P. et al. (2023). Anti-viral and Anti-inflammatory Isoflavonoids from Ukrainian Iris aphylla Rhizomes: Structure-Activity Relationship Coupled with ChemGPS-NP Analysis. Planta Medica, 89 (11), 1063–1073. https://doi.org/10.1055/a-2063-5265
- Mykhailenko, O., Kovalyov, V., Kovalyov, S., Krechun, A. (2017). Isoflavonoids from the rhizomes of Iris hungarica and antibacterial activity of the dry rhizomes extract. Ars Pharmaceutica (Internet), 58 (1), 39–45. https://doi.org/10.30827/ars.v58i1.5919
- Kerimova, G. F., Rybak, V. A., Krechun, А. V., Kovalev, V. М. (2020). Study of anabolic activity of dry extracts of leaves and rootstalks of iris hungarica in intact animals. Fitoterapia, 2 (2), 50–55. https://doi.org/10.33617/2522-9680-2020-2-50
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Viktoria Rybak, Gunel Kerimova, Dmytro Lytkin, Olha Mykhailenko

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






