The content and stability of ascorbic acid in commercial food supplements
DOI:
https://doi.org/10.15587/2519-4852.2024.313637Keywords:
ascorbic acid, food supplements, shelf life, EstoniaAbstract
Ascorbic acid is a well-known compound found in many vegetables and fruits. In medical practice, it is used in the composition of many drugs and food supplements. Taking into account the different quality of production of food supplements on the one hand and the lability of ascorbic acid on the other, it is important to analyze the real content of ascorbic acid in food supplements containing it.
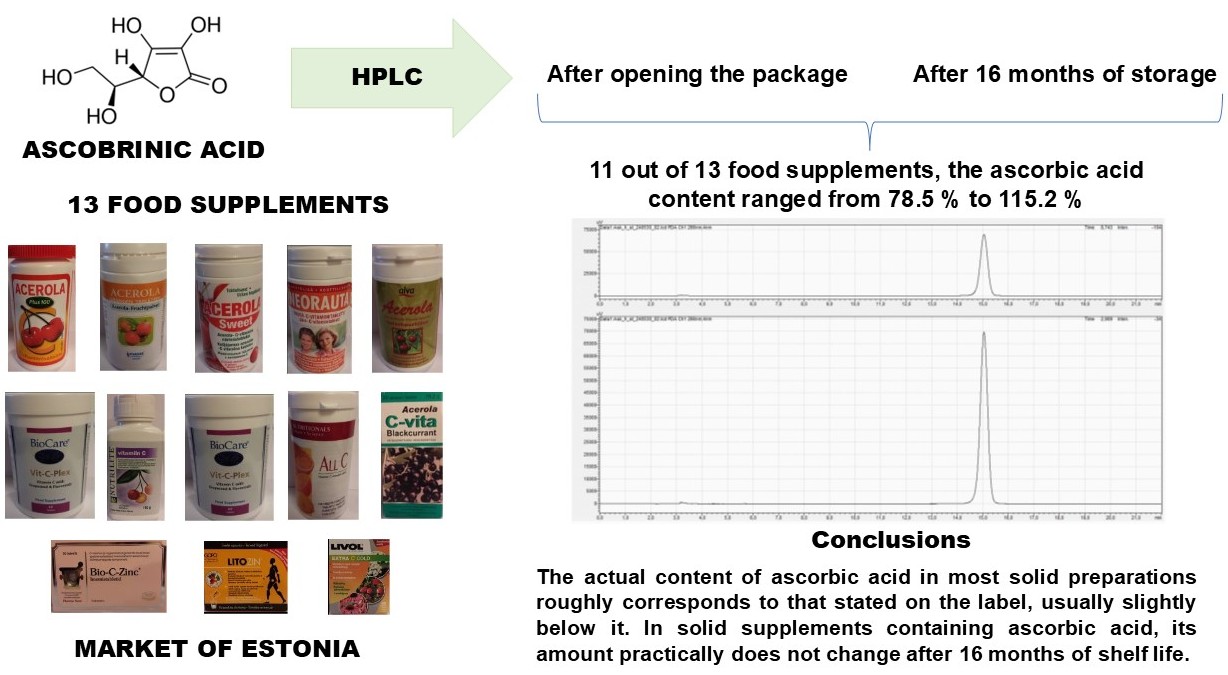
The aim. The aim of the study was to compare the content of ascorbic acid in food supplements from different manufacturers to establish the stability of ascorbic acid over time and the relationship between the content of ascorbic acid and the shelf life of food supplements, as well as to investigate the relationship between the stability of ascorbic acid and the nature of its origin.
Materials and methods. 13 preparations of food supplements containing ascobrinic acid, which were sold in Estonian pharmacies or online environments at the time of the work, were analysed. Quantitative analysis of ascorbic acid was performed using the HPLC method.
Research results. It was found that in 11 out of 13 food supplements, the ascorbic acid content ranged from 78.5 % to 115.2 % of the nominal. For two samples, the ascorbic acid content was very low compared to that provided by the manufacturer (54.6-56.3 %). The content of ascorbic acid in three preparations does not meet the standards set by the European Commission (from -20 % to +50 %). Аfter 16 months of storage, a statistically significant change in the content of ascorbic acid occurred only in four samples, in which its content decreased (p<0.05) by about 7-21 mg. The ascorbic acid content in the other samples did not change for 16 months after storage of the opened packages. Statistical analysis of the data showed that the relative amount of ascorbic acid present in the preparation relative to the nominal was related to the origin of the substance, whereas the content of ascorbic acid of natural origin in preparations relative to the nominal was significantly lower than in preparations with a synthetic active substance.
Conclusions. The actual content of ascorbic acid in most solid preparations roughly corresponds to that stated on the label, usually slightly below it. In solid supplements containing ascorbic acid, its amount practically does not change after 16 months of shelf life. In preparations containing ascorbic acid of natural origin, it is found relatively less than in preparations containing synthetic ascorbic acid
Supporting Agency
- European Union in the MSCA4Ukraine project “Design and development of 3D-printed medicines for bioactive materials of Ukrainian and Estonian medicinal plants origin” [ID number 1232466]
References
- Yin, X., Chen, K., Cheng, H., Chen, X., Feng, S., Song, Y., Liang, L. (2022). Chemical Stability of Ascorbic Acid Integrated into Commercial Products: A Review on Bioactivity and Delivery Technology. Antioxidants, 11 (1), 153. https://doi.org/10.3390/antiox11010153
- Pilarski, B., Wyrzykowski, D., Młodzianowski, J. (2023). A New Approach for Studying the Stability and Degradation Products of Ascorbic acid in Solutions. Journal of Solution Chemistry, 52 (6), 639–657. https://doi.org/10.1007/s10953-023-01260-9
- Shelke, O., Susarla, K. P. C., Bankar, M. (2024). Understand the Stabilization Engineering of Ascorbic Acid, Mapping the Scheme for Stabilization, and Advancement. AAPS PharmSciTech, 25 (6). https://doi.org/10.1208/s12249-024-02882-y
- Krečak, I., Babić, G., Skelin, M. (2022). Scurvy. Acta dermatovenerologica Croatica: ADC, 30 (1), 59–60.
- Bhoot, H. R., Zamwar, U. M., Chakole, S., Anjankar, A. (2023). Dietary Sources, Bioavailability, and Functions of Ascorbic Acid (Vitamin C) and Its Role in the Common Cold, Tissue Healing, and Iron Metabolism. Cureus, 15 (11), e49308. https://doi.org/10.7759/cureus.49308
- Carr, A., Maggini, S. (2017). Vitamin C and Immune Function. Nutrients, 9 (11), 1211. https://doi.org/10.3390/nu9111211
- Nowak, D. (2021). Vitamin C in Human Health and Disease. Nutrients, 13 (5), 1595. https://doi.org/10.3390/nu13051595
- Wang, J. L. (2019). Vitamin C in Human Health and Disease. Springer Dordrecht, 184. https://doi.org/10.1007/978-94-024-1713-5
- Doseděl, M., Jirkovský, E., Macáková, K., Krčmová, L., Javorská, L., Pourová, J. et al. (2021). Vitamin C – Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients, 13 (2), 615. https://doi.org/10.3390/nu13020615
- Abdullah, M., Jamil, R. T., Attia, F. N. (2023). Vitamin C (Ascorbic Acid) Treasure Island: StatPearls Publishing. Available at: https://www.ncbi.nlm.nih.gov/books/NBK499877/
- Granger, M., Eck, P. (2018). Dietary Vitamin C in Human Health. Advances in food and nutrition research, 281–310. https://doi.org/10.1016/bs.afnr.2017.11.006
- Paciolla, C., Fortunato, S., Dipierro, N., Paradiso, A., De Leonardis, S., Mastropasqua, L., de Pinto, M. C. (2019). Vitamin C in Plants: From Functions to Biofortification. Antioxidants, 8 (11), 519. https://doi.org/10.3390/antiox8110519
- Belwal, T., Devkota, H. P., Hassan, H. A., Ahluwalia, S., Ramadan, M. F., Mocan, A., Atanasov, A. G. (2018). Phytopharmacology of Acerola (Malpighia spp. ) and its potential as functional food. Trends in Food Science & Technology, 74, 99–106. https://doi.org/10.1016/j.tifs.2018.01.014
- Poletto, P., Álvarez-Rivera, G., López, G.-D., Borges, O. M. A., Mendiola, J. A., Ibáñez, E., Cifuentes, A. (2021). Recovery of ascorbic acid, phenolic compounds and carotenoids from acerola by-products: An opportunity for their valorization. LWT, 146, 111654. https://doi.org/10.1016/j.lwt.2021.111654
- Carneiro Ferreira, I., Pereira da Silva, V., Vilvert, J. C., França Souza, F., Freitas, S. T., dos Santos Lima, M. (2021). Brazilian varieties of acerola (Malpighia emarginataDC.) produced under tropical semi‐arid conditions: Bioactive phenolic compounds, sugars, organic acids, and antioxidant capacity. Journal of Food Biochemistry, 45 (8). https://doi.org/10.1111/jfbc.13829
- Olędzki, R., Harasym, J. (2024). Acerola (Malpighia emarginata) Anti-Inflammatory Activity – A Review. International Journal of Molecular Sciences, 25 (4), 2089. https://doi.org/10.3390/ijms25042089
- Šmíd, J., Kalousová, M., Mandák, B., Houška, J., Chládová, A., Pinedo, M., Lojka, B. (2017). Morphological and genetic diversity of camu-camu [Myrciaria dubia (Kunth) McVaugh] in the Peruvian Amazon. PLOS ONE, 12(6), e0179886. https://doi.org/10.1371/journal.pone.0179886
- CAMU CAMU (Myrciaria dubia) (2006). FAO. Available at: https://www.ipcinfo.org/fileadmin/user_upload/inpho/InfoSheet_pdfs/CAMU_CAMU.pdf
- Conceição, N., Albuquerque, B. R., Pereira, C., Corrêa, R. C. G., Lopes, C. B., Calhelha, R. C. et al. (2019). By-Products of Camu-Camu [Myrciaria dubia (Kunth) McVaugh] as Promising Sources of Bioactive High Added-Value Food Ingredients: Functionalization of Yogurts. Molecules, 25 (1), 70. https://doi.org/10.3390/molecules25010070
- García-Chacón, J. M., Marín-Loaiza, J. C., Osorio, C. (2023). Camu Camu (Myrciaria dubia (Kunth) McVaugh): An Amazonian Fruit with Biofunctional Properties–A Review. ACS Omega, 8 (6), 5169–5183. https://doi.org/10.1021/acsomega.2c07245
- Cunha-Santos, E. C. E., Viganó, J., Neves, D. A., Martínez, J., Godoy, H. T. (2019). Vitamin C in camu-camu [Myrciaria dubia (H.B.K.) McVaugh]: evaluation of extraction and analytical methods. Food Research International, 115, 160–166. https://doi.org/10.1016/j.foodres.2018.08.031
- European Pharmacopoeia (2022). Strasbourg: Council of Europe.
- Medveckienė, B., Kulaitienė, J., Jarienė, E., Vaitkevičienė, N., Hallman, E. (2020). Carotenoids, Polyphenols, and Ascorbic Acid in Organic Rosehips (Rosa spp.) Cultivated in Lithuania. Applied Sciences, 10 (15), 5337. https://doi.org/10.3390/app10155337
- Butkevičiūtė, A., Urbštaitė, R., Liaudanskas, M., Obelevičius, K., Janulis, V. (2022). Phenolic Content and Antioxidant Activity in Fruit of the Genus Rosa L. Antioxidants, 11 (5), 912. https://doi.org/10.3390/antiox11050912
- Kask, M., Meos, A., Raal, A. (2013). Askorbiinhappe sisaldusest apelsinimahlas. Eesti Rohuteadlane, 2, 13–15.
- Karaklajic-Stajic, Z., Tomic, J., Pesakovic, M., Paunovic, S. M., Stampar, F., Mikulic-Petkovsek, M. et al. (2023). Black Queens of Fruits: Chemical Composition of Blackberry (Rubus subg. rubus Watson) and Black Currant (Ribes nigrum L.) Cultivars Selected in Serbia. Foods, 12 (14), 2775. https://doi.org/10.3390/foods12142775
- Cortez, R. E., Gonzalez de Mejia, E. (2019). Blackcurrants (Ribes nigrum): A Review on Chemistry, Processing, and Health Benefits. Journal of Food Science, 84 (9), 2387–2401. https://doi.org/10.1111/1750-3841.14781
- Meos, A., Zaharova, I., Kask, M., Raal, A. (2017). Content of Ascorbic Acid in Common Cowslip (Primula veris L.) Compared to Common Food Plants and Orange Juices. Acta Biologica Cracoviensia s. Botanica, 59 (1), 113–120. https://doi.org/10.1515/abcsb-2016-0020
- European Commission. Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the approximation of the laws of the Member States relating to food supplements (2002). Off J Eur Communities, L183/51. http://data.europa.eu/eli/dir/2002/46/oj
- In brief: What are dietary supplements? (2024). Cologne: Institute for Quality and Efficiency in Health Care (IQWiG). InformedHealth.org. Available at: https://www.ncbi.nlm.nih.gov/books/NBK279425/
- Regulation of Dietary Supplements: Background and Issues for Congress (2021). CRS Report. Available at: https://sgp.fas.org/crs/misc/R43062.pdf
- FDA 101: Dietary Supplements (2022). FDA. Available at: https://www.fda.gov/consumers/consumer-updates/fda-101-dietary-supplements
- Dietary Supplements Market Size by Ingredient Type (Vitamins, Minerals, Enzymes, Probiotics, Fibers & Specialty Carbohydrates, Botanicals, Proteins & Amino Acids, Omega Fatty Acids, and Others), Application (Energy & Weight Management, Bone & Joint Health, Immunity, Diabetes, Lungs, Skin/Hair, Mental Health, Menopause, Prenatal Health, Anti-Aging, Insomnia, Sexual Health, Anti-Cancer, Cardiac Health, Gastrointestinal Health, General Health, and Others), Form Type, Distribution Channel, Regions, Global Industry Analysis, Share, Growth, Trends, and Forecast 2022 to 2030 (2024). Available at: https://www.thebrainyinsights.com/report/dietary-supplements-market-12888
- Lentjes, M. A. H. (2018). The balance between food and dietary supplements in the general population. Proceedings of the Nutrition Society, 78 (1), 97–109. https://doi.org/10.1017/s0029665118002525
- Saini, P., Ahmed, M., Iqbal, U., Yadav, N.; Rajakumari, R., Thomas, S. (Eds.) (2024). Challenges in Stability and Safety Evaluation of Nutraceutical and Nanonutraceutical Formulations. Handbook of Nutraceuticals. Cham: Springer. https://doi.org/10.1007/978-3-030-69677-1_16-1
- Dey, P, Jain, N, Nagaich, U. (2018). Nutraceuticals: an overview of regulations. International Journal of Pharmaceutical and Life Sciences, 9, 5762–5766.
- Zhang, F. F., Barr, S. I., McNulty, H., Li, D., Blumberg, J. B. (2020). Health effects of vitamin and mineral supplements. BMJ, m2511. https://doi.org/10.1136/bmj.m2511
- Rao, K. S. (2018). Safety Assessment of Nutraceuticals. Biology, Engineering, Medicine and Science Reports, 3 (2), 70–72. https://doi.org/10.5530/bems.3.2.9
- White, C. M. (2020). Dietary Supplements Pose Real Dangers to Patients. Annals of Pharmacotherapy, 54 (8), 815–819. https://doi.org/10.1177/1060028019900504
- Giannakourou, M. C., Taoukis, P. S. (2021). Effect of Alternative Preservation Steps and Storage on Vitamin C Stability in Fruit and Vegetable Products: Critical Review and Kinetic Modelling Approaches. Foods, 10 (11), 2630. https://doi.org/10.3390/foods10112630
- Raal, A., Nisuma, K., Meos, A. (2018). Pinus sylvestris L. and other conifers as natural sources of ascorbic acid. Journal of Pharmacy & Pharmacognosy Research, 6 (1), 89–95. https://doi.org/10.56499/jppres17.287_6.2.89
- Golubitskii, G. B., Budko, E. V., Basova, E. M., Kostarnoi, A. V., Ivanov, V. M. (2007). Stability of ascorbic acid in aqueous and aqueous-organic solutions for quantitative determination. Journal of Analytical Chemistry, 62 (8), 742–747. https://doi.org/10.1134/s1061934807080096
- Harvey, D. Critical Values for Dixon's Q-Test. Available at: https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Analytical_Chemistry_Volume_I_(Harvey)/10%3A_Appendix/10.06%3A_Critical_Values_for_Dixon's_Q-Test
- Siddiqui, M. R., AlOthman, Z. A., Rahman, N. (2017). Analytical techniques in pharmaceutical analysis: A review. Arabian Journal of Chemistry, 10, S1409–S1421. https://doi.org/10.1016/j.arabjc.2013.04.016
- Saisana, M.; Maggino, F. (Ed.) (2023). Analysis of Variance. Encyclopedia of Quality of Life and Well-Being Research. Cham: Springer, 183–187. https://doi.org/10.1007/978-3-031-17299-1_83
- Guidance Document Tolerances: Simplified Summary Table (2012). The European Commission. Available at: https://food.ec.europa.eu/system/files/2016-10/labelling_nutrition-vitamins_minerals-guidance_tolerances_summary_table_012013_en.pdf
- Bae, D.-H., Gholam Azad, M., Kalinowski, D. S., Lane, D. J. R., Jansson, P. J., Richardson, D. R. (2020). Ascorbate and Tumor Cell Iron Metabolism: The Evolving Story and Its Link to Pathology. Antioxidants & Redox Signaling, 33 (12), 816–838. https://doi.org/10.1089/ars.2019.7903
- Ševeljova, O. (2012). Vitamiin C sisaldus ja selle muutus säilitamisel ravimpreparaatides. Tartu: Proviisoriõppe uurimistöö, Tartu Ülikool.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Raal Ain, Andres Meos, Agne Vutt, Herman Kirsimäe, Тetiana Ilina, Alla Kovaleva, Oleh Koshovyi

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






