Розробка складу дерматологічного засобу у формі крему з вилученнями надземної частини леспедези двоколірної
DOI:
https://doi.org/10.15587/2519-4852.2025.322855Ключові слова:
надземна частина леспедези двоколірної, фенольні сполуки, дерматологічний крем, екстракти леспедези двоколірної, протизапальна активність, технологія ліківАнотація
Мета роботи: Обґрунтування складу емульсійного крему з екстрактами леспедези двоколірної.
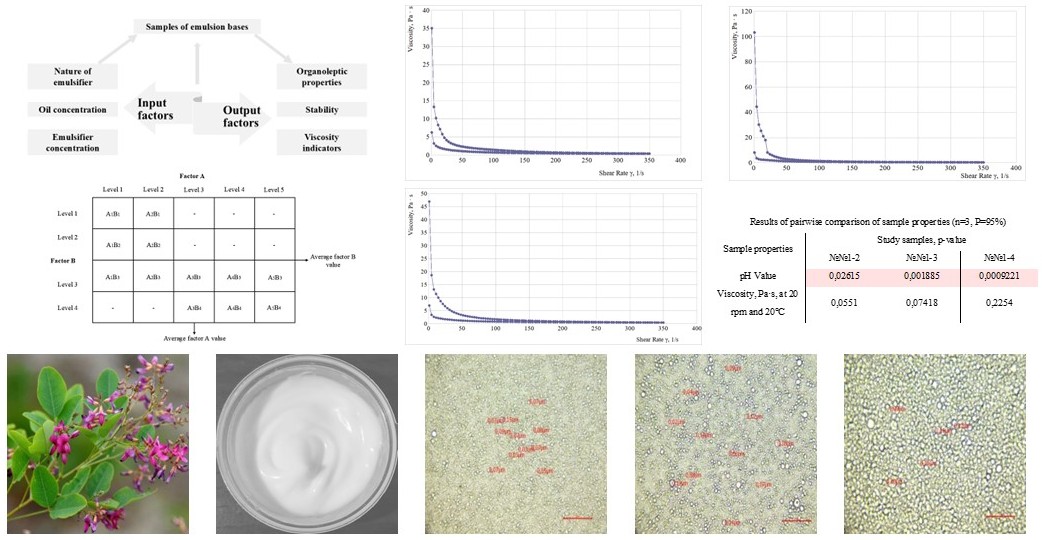
Матеріали і методи: В розробці емульсійної основи крему використовували олію кукурудзяну (Україна), пропленгліколь (Німеччина), воду очищену, емульгатори: ксильянс (Франція), проліпід 141 (США) і ламекрем (Франція). До складу емульсійної основи вводили олійний і рідкий спиртовий екстракт наземної частини леспедези двоколірної, які отримували з надземної частина леспедези двоколірної, заготовленої у Ботанічному саді ЛНУ ім. Івана Франка (Львів, Україна) у фазу цвітіння. В дослідженнях використовували фізико-хімічні (значення рН, ідентифікація та кількісний вміст БАС), фармакотехнологічні (термо- та колоїдна стабільність, структурно-механічні властивості та дисперсний аналіз) методи дослідження. Протизапальну активність дослідних зразків вивчали на моделі опікової рани на безпородних статевозрілих щурах самцях. Як препарати порівняння використовували лікарські засоби мазь Пантенол, «Хемофарм», АД, Сербія, серія 138CLA та мазь Календули, ТОВ «Аптека Павлова», Україна, серія 23.0823.
Результати. Експериментальні дослідження органолептичних, фізико-хімічних і структурно-механічних властивостей зразків емульсійних основ показали їх залежність від концентрації олійної фази, складу і концентрації комплексних емульгаторів ксильянс, проліпід 141 та ламекрем. Встановлено, що емульсійна основа крему, яка містить 15 % олії кукурудзяної, 7 % комплексного емульгатору ксильянс, 5 % пропіленгліколю та води очищеної до 100 має задовільні структурно-механічні властивості, необхідну дисперсність і гомогенний розподіл часток олійної фази в водному дисперсійному середовищі, витримує тест на термо- і колоідну стабільність і може бути використана для розробки дерматологічного крему з екстрактами леспедези. Показано, що введення екстрактів до складу розробленої основи не впливає на стабільність, структурно-механічні властивості основи та вміст БАС. Дослідження протизапальної активності крему з комплексом біологічно активних сполук олійного і спиртового екстрактів наземної частини леспедези двоколірної на моделі опікової рани показали зменшення ознак запалення без ознак приєднання інфекційного процесу у лабораторних тварин з загоєнням ран на 26 добу дослідження.
Висновки. Експериментально обґрунтовано склад крему з олійним і спиртовим екстрактом леспедези, який завдяки комплексу БАС володіє більш широким спектром фармакологічної активності. Розроблено склад емульсійної основи крему з олією кукурудзяною, емульгатором ксильянс, пропіленгліколем та водою очищеною. Показано, що введення екстрактів до складу розробленої основи не впливає на його фармакотехнологічні властивості, а кількісний вміст біологічно активних сполук в кремі відповідає їх вмісту в екстрактах в перерахунку на концентрацію екстрактів в МЛФ, що є підтвердженням сумісності БАС з допоміжними речовинами Встановлено, що крем, який містить комплекс БАС леспедези двоколірної за протизапальною активністю знаходиться на рівні препаратів порівняння мазь Пантенол та мазь Календули
Посилання
- Yazarlu, O., Iranshahi, M., Kashani, H. R. K., Reshadat, S., Habtemariam, S., Iranshahy, M., Hasanpour, M. (2021). Perspective on the application of medicinal plants and natural products in wound healing: A mechanistic review. Pharmacological Research, 174, 105841. https://doi.org/10.1016/j.phrs.2021.105841
- Ahuja, A., Gupta, J., Gupta, R. (2021). Miracles of Herbal Phytomedicines in Treatment of Skin Disorders: Natural Healthcare Perspective. Infectious Disorders – Drug Targets, 21 (3), 328–338. https://doi.org/10.2174/1871526520666200622142710
- Ullah, S. (2017). Methanolic extract from Lespedeza bicolor: potential candidates for natural antioxidant and anticancer agent. Journal of Traditional Chinese Medicine, 37 (4), 444–451. https://doi.org/10.1016/s0254-6272(17)30150-4
- Kim, S. J., Kim, D. W. (2007). Antoxidative activity of hot water and ethanol extracts of Lespedeza cuneata seeds. Korean Journal of Food Preservation, 14, 332–335.
- Ren, C., Li, Q., Luo, T., Betti, M., Wang, M., Qi, S. et al. (2023). Antioxidant Polyphenols from Lespedeza bicolor Turcz. Honey: Anti-Inflammatory Effects on Lipopolysaccharide-Treated RAW 264.7 Macrophages. Antioxidants, 12 (10), 1809. https://doi.org/10.3390/antiox12101809
- Lee, S. J., Hossaine, M. D. A., Park, S. C. (2016). A potential anti-inflammation activity and depigmentation effect of Lespedeza bicolor extract and its fractions. Saudi Journal of Biological Sciences, 23 (1), 9–14. https://doi.org/10.1016/j.sjbs.2015.01.016
- Thuy, N. T. T., Lee, J.-E., Yoo, H. M., Cho, N. (2019). Antiproliferative Pterocarpans and Coumestans from Lespedeza bicolor. Journal of Natural Products, 82 (11), 3025–3032. https://doi.org/10.1021/acs.jnatprod.9b00567
- Nam, S. H. (2023) Evalution of the anti-caries effect of Lespedeza cuneata extract against Streptococcus mutans. Georgian Med News, 3 (38), 19–22.
- Hong, H.-J., Son, N.-R., Yang, W.-Y., Lee, J.-M., Kim, J.-H., Jang, S.-M., Nam, S.-H. (2018). Antibacterial and antifungal activities of Lespedeza cuneata extract against Candida albicans. Biomedical Research, 29 (20). https://doi.org/10.4066/biomedicalresearch.29-18-1080
- Woo, H. S., Lee, K. H., Park, K. H., Kim, D. W. (2024). Flavonoids Derived from the Roots of Lespedeza bicolor Inhibit the Activity of SARS-CoV Papain-like Protease. Plants, 13 (23), 3319. https://doi.org/10.3390/plants13233319
- Leti, M., Daunes-Marion, S., Leveque, M. (2020). WO2020020791A1; WIPO (PCT). Lespedeza capitata extract for use in the field of hair care. Available at: https://patents.google.com/patent/WO2020020791A1/en
- Seong, J. S., Xuan, S. H., Park, S. H., Lee, K. S., Park, Y. M., Park, S. N. (2017). Antioxidative and Antiaging Activities and Component Analysis of Lespedeza cuneata G. Don Extracts Fermented with Lactobacillus pentosus. Journal of Microbiology and Biotechnology, 27 (11), 1961–1970. https://doi.org/10.4014/jmb.1706.06028
- Deng, F., Chang, J., Zhang, J.-S. (2007). New flavonoids and other constituents from Lespedeza cuneata. Journal of Asian Natural Products Research, 9 (7), 655–658. https://doi.org/10.1080/10286020600979894
- Kiselyova, K. E., Bevz, N. Yu., Mykhailenko, O. O., Yaromiy, M. V., Vyshnevska, L. I. (2024). The substantiation of the choice of an extractant for obtaining extractions of the overground part of Lespedeza bicolor. News of Pharmacy, 107 (1), 58–65. https://doi.org/10.24959/nphj.24.126
- Kiselyova, K. E., Bevs, N. Yu., Mykhaylenko, O. O., Vishnevska, L. I.; Bessarabov, V., Lubenets, V. (Eds.) (2023). Justification of the conditions for obtaining the oil extract of Lespedecia bicolor. Chemical and biopharmaceutical technologies. Tallinn: Nordic Sci Publisher, 336–342.
- Kiselyova, K., Osolodchenko, T., Vishnevska, L. (2024). Study of the antibacterial action of Lespedecia bicolor extracts and cream based on them. Annals of the Mechnikov Institute, 2, 69–73. https://doi.org/10.5281/zenodo.11638092
- Kiselyova, K. E., Vishnevska, L. I. (2024). Study of the anti-inflammatory activity of extracts of Lespedecia bicolor. Industry 4.0: modern directions of development of the pharmaceutical industry. Kharkiv: Publishing House of the National Academy of Sciences, 51–53.
- Herbig, M. E., Evers, D.-H., Gorissen, S., Köllmer, M. (2023). Rational Design of Topical Semi-Solid Dosage Forms-How Far Are We? Pharmaceutics, 15 (7), 1822. https://doi.org/10.3390/pharmaceutics15071822
- Dragicevic, N., Maibach, H. I. (2021). Percutaneous Absorption: Drugs, Cosmetics, Mechanisms, Methods. Boca Raton: CRC Press, 1008. https://doi.org/10.1201/9780429202971
- Grégoire, S., Ribaud, C., Benech, F., Meunier, J. R., Garrigues-Mazert, A., Guy, R. H. (2009). Prediction of chemical absorption into and through the skin from cosmetic and dermatological formulations. British Journal of Dermatology, 160 (1), 80–91. https://doi.org/10.1111/j.1365-2133.2008.08866.x
- Dias, M., Hadgraft, J., Lane, M. (2007). Influence of membrane–solvent–solute interactions on solute permeation in skin. International Journal of Pharmaceutics, 340 (1-2), 65–70. https://doi.org/10.1016/j.ijpharm.2007.03.030
- Oliveira, G., Hadgraft, J., Lane, M. E. (2012). The influence of volatile solvents on transport across model membranes and human skin. International Journal of Pharmaceutics, 435 (1), 38–49. https://doi.org/10.1016/j.ijpharm.2012.05.037
- Herbig, M. E. (2022). Topical Drug Delivery and the Role of Excipients. Chimica Oggi-Chemistry Today, 40, 34–37.
- ST-N MOZU 42-3.0:2011 «Likarski zasoby. Farmatsevtychna rozrobka (ICH Q8)» (2011). Kyiv: Ministry of Health of Ukraine, 13.
- Lane, M. E., Hadgraft, J., Oliveira, G., Vieira, R., Mohammed, D., Hirata, K. (2012). Rational formulation design. International Journal of Cosmetic Science, 34 (6), 496–501. https://doi.org/10.1111/j.1468-2494.2012.00747.x
- Derzhavna Farmakopeia Ukrainy. Vol. 1 (2015). Kharkiv: DP «Ukrainskyi naukovyi farmakopeinyi tsentr yakosti likarskykh zasobiv», 1128.
- Stefanova, O. V. (Ed.) (2001). Doklinichni doslidzhennia likarskykh zasobiv. Kyiv: Avicenna, 528.
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes (2010). Official Journal of the European Union, L276, 33–79.
- Medicines. Good laboratory practice (2009). Kyiv: Ministry of Health of Ukraine, 27.
- Yakovleva, L. V., Tkacheva, O. V., Butko, I. O., Larianovska, Yu. B. (2013). Experimental study of new drugs for local treatment of wounds. Kharkiv: NFaU Publishing House, 52.
- Trapella, C., Rizzo, R., Gallo, S., Alogna, A., Bortolotti, D., Casciano, F. et al. (2018). HelixComplex snail mucus exhibits pro-survival, proliferative and pro-migration effects on mammalian fibroblasts. Scientific Reports, 8 (1). https://doi.org/10.1038/s41598-018-35816-3
- Tkachova, O. V. (2014). Pharmacological study of new drugs developed on the basis of natural substances and intended for the local treatment of wound healing process. [Extended abstract of PhD thesis].
- Indrayan, A., Malhotra, K. R. (2018). Medical biostatistics. Boca Raton: CRC Press, 685.
- Roberts, M. S., Cheruvu, H. S., Mangion, S. E., Alinaghi, A., Benson, H. A. E., Mohammed, Y. et al. (2021). Topical drug delivery: History, percutaneous absorption, and product development. Advanced Drug Delivery Reviews, 177, 113929. https://doi.org/10.1016/j.addr.2021.113929
- Kukhtenko, H., GladukhIe, Y., Kukhtenko, O., Soldatov, D. (2017). Influence of Excipients on the Structural and Mechanical Properties of Semisolid Dosage Forms. Asian Journal of Pharmaceutics, 11 (3), 575–578.
- Bulyha, L. O., Chernykh, V. P., Shtryhol, S. Iu., Movchan, B. O., Butko, Ya. O. (2015). Eksperymentalne doslidzhennia ranozahoiuvalnoi dii heliu z nanochastynkamy sribla ta hliukozaminom. Pharmacology and medicinal toxicology, 2 (43), 49–54.
- Mayevsky, O. E., Mironov, E. V. (2015). Changes in the skin after thermal burns (literature review). Biomedical and biosocial anthropology, 25, 218–220.
##submission.downloads##
Опубліковано
Як цитувати
Номер
Розділ
Ліцензія
Авторське право (c) 2025 Kate Kiselyova, Tetiana Yudkevych, Liubov Bodnar, Mariia Skybitska, Liliia Vyshnevska, Tetiana Yudkevych, Liudas Ivanauskas, Olha Mykhailenko, Oleksandr Kukhtenko, Victoriya Georgiyants

Ця робота ліцензується відповідно до Creative Commons Attribution 4.0 International License.
Наше видання використовує положення про авторські права Creative Commons CC BY для журналів відкритого доступу.








