HTA in Germany: basics in practice and education
DOI:
https://doi.org/10.15587/2519-4852.2025.326977Keywords:
healthcare, health economy, health assessment, health technology, health technology assessment (HTA), HTA curriculum, HTA authorities, competence grid, HTA stakeholders, target groups in HTAAbstract
The aim. The aim of the investigation was to elucidate the HTA system's development process and its educational component by studying the basic functions and options of services that are responsible for its functioning in Germany.
Material and methods. Official sites of government and non-government non-profit organizations and government publications in specialized open sources have been used. Research methods include mostly descriptive approaches and methods for collecting data: observation, generalization data, graphics and methods for analyzing data: qualitative content analysis.
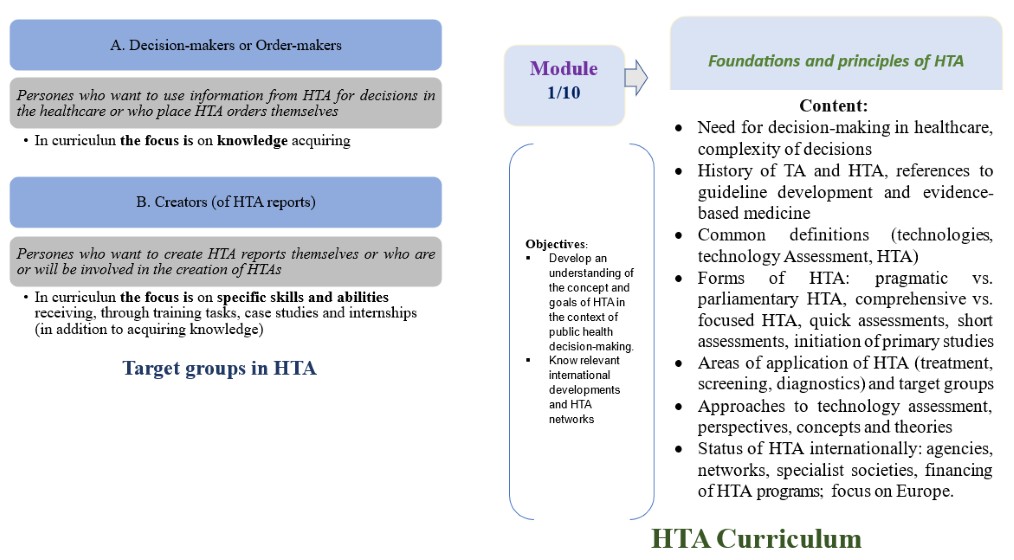
Results. The creation of the HTA system in Germany has been ongoing since 1994. State institutions responsible for HTA have changed, and their areas of responsibility and control have been reformatted. Today, 5 institutions/organizations that ensure the effective functioning of the HTA in Germany are presented. A basic training program aimed at training executors (reporters) and decision-makers is presented. A grid of 7 basic competencies in HTA education in Germany is presented. The program is presented in 10 modules, which are grouped by the authors into three cycles: Fundamental (Modules 1-6), Methodological (M. 7-9) and final cycles (M. 10).
Conclusions. To build or improve the HTA system, attention should be paid to the periodicity of updating information, which will contribute to a more accurate identification (satisfaction) of the needs of the country's HTA, both from the standpoint of implementation and ensuring applied functioning, and for the educational component of the HTA process. Constant review and assessment of the need/basic scope of the national HTA and stakeholders. The best way to do this is to study existing models and mechanisms of HTA in countries with the most developed and advanced healthcare systems
References
- OECD. Available at: https://www.oecd.org/
- Situation Report – 122. Coronavirus disease 2019 (COVID-19) (2020). Available at: https://www.who.int/publications/m/item/situation-report---122
- Szczepura, A., Kankaanpää, J. (1994). Interests in Health Care Technology Assessment (HCTA) and HCTA training needs in eight European countries: Comett-assess. Social Science & Medicine, 38 (12), 1679–1688. https://doi.org/10.1016/0277-9536(94)90070-1
- EUnetHTA. HTA Core Model. Available at: https://learning.eupati.eu/mod/page/view.php?id=956
- Fontrier, A.-M., Visintin, E., Kanavos, P. (2021). Similarities and Differences in Health Technology Assessment Systems and Implications for Coverage Decisions: Evidence from 32 Countries. PharmacoEconomics – Open, 6 (3), 315–328. https://doi.org/10.1007/s41669-021-00311-5
- Vreman, R. A., Mantel-Teeuwisse, A. K., Hövels, A. M., Leufkens, H. G. M., Goettsch, W. G. (2020). Differences in Health Technology Assessment Recommendations Among European Jurisdictions: The Role of Practice Variations. Value in Health, 23 (1), 10–16. https://doi.org/10.1016/j.jval.2019.07.017
- Curriculum EbM Ärztlicher Fortbildungskatalog Evidenzbasierte Medizin (2005). Deutsches Netzwerk Evidenzbasierte Medizin (DNEbM e.V.); Ärztliches Zentrum für Qualität in der Medizin (ÄZQ), Hrsg. Available at: https://www.bundesaerztekammer.de/fileadmin/user_upload/_old-files/downloads/CurrEBM.pdf
- Health Economics and Health Technology Assessment MSc/PgDip/PgCert: Online distance learning. Postgraduate Taught. University of Glasgow. Available at: http://www.gla.ac.uk/postgraduate/taught/healthtechnologyassessment
- Health Technology Assessment and Decision Science Program. Doctoral Program in Health Technology Assessment. UMIT – University for Health Sciences, Medical Informatics and Technology. HTADS – Program on Health Technology Assessment & Decision Sciences HTADS. Available at: https://www.umit-tirol.at/page.cfm?vpath=academy/htads-kurse&switchLocale=de_AT
- Hoxhaj, I., Castagna, C., Calabrò, G. E., Boccia, S. (2022). HTA Training for Healthcare Professionals: International Overview of Initiatives Provided by HTA Agencies and Organizations. Frontiers in Public Health, 10. https://doi.org/10.3389/fpubh.2022.795763
- GMS Health Innovation and Technologies. Das Curriculum Health Technology Assessment (HTA), Version 2.0. Available at: https://www.egms.de/static/en/journals/hta/2017-13/hta000129.shtml
- Douw, K., Vondeling, H., Bakketeig, Leiv. S., Gabbay, J., Hansen, N. W., Kristensen, F. B. (2002). HTA education and training in Europe. International Journal of Technology Assessment in Health Care, 18 (4), 808–819. https://doi.org/10.1017/s0266462302000612
- Health Technology Assessment (HTA) Training Program. International Society for Pharmacoeconomics and Outcomes Research. ISPOR. Available at: https://www.ispor.org/education-training/learning-lab/health-technology-assessment
- University of Birmingham. Public Health (Health Technology Assessment) MPH/Diploma. Available at: http://www.birmingham.ac.uk/postgraduate/courses/taught/med/public-health-tech-assessment.aspx.
- DIMDI. Federal Institute for Drugs and Medical Devices (BfArM) Office in Cologne. Available at: https://www.bfarm.de/EN/Home/_node.html
- Fasseeh, A. N., Saragih, S. M., Hayek, N., Brodovska, S., Ismail, A., ElShalakani, A. et al. (2022). Impact of health technology assessment implementation with a special focus on middle-income countries. Health Policy and Technology, 11 (4), 100688. https://doi.org/10.1016/j.hlpt.2022.100688
- Health technology assessment. Available at: https://www.who.int/health-topics/health-technology-assessment#tab=tab_1
- Health Technology Assessment process: Fundamentals. Available at: https://toolbox.eupati.eu/resources/health-technology-assessment-process-fundamentals/
- Carroll, C., Beecroft, C., Miller, L. (2014). The Future of Education in HTA and Health Economics. Value in Health, 17 (7), A436. https://doi.org/10.1016/j.jval.2014.08.1123
- EUnetHTA 21 Servicevertrag endet (2021). SKC. Available at: https://skc-beratung.de/de/insights/blog/2023/09/EUnetHTA_21_servicevertrag_endet.php
- INAHTA Members List. Available at: https://www.inahta.org/members/members_list/
- Organizational Members. HTAi. Available at: https://htai.org/membership/organizational-members/
- Filiniuk, O., Babenko, M., Kosyachenko, K., Sucu, R. (2023). Current approaches of health technologies introduction in Ukrainian hospitals. ScienceRise: Pharmaceutical Science, 5 (45), 16–23. https://doi.org/10.15587/2519-4852.2023.289683
- Zaliska, O., Piniazhko, O., Vashchenko, O., Brezden, O. (2019). Analysis of education and training opportunities in HE/PE/HTA in CEE consortium countries. Value in Health, 22, 8–22. https://doi.org/10.1016/j.jval.2019.09.2241
- Kotvitskа, A. А., Nemchenko, A. S., Nazarkina, V. N. (2020). The relevance of training specialists in the Health Technology Assessment in the world and Ukraine. Pharmacia, 67 (4), 295–301. https://doi.org/10.3897/pharmacia.67.e54777
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Maryna Podgaina

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.







