Dose-dependent effects of mitomycin С in non-healing wound modeling
DOI:
https://doi.org/10.15587/2519-4852.2025.338113Keywords:
non-healing wound, chronic wound, mitomycin C, re-epithelialization, scar, fibrous tissue, keratinocytes, endothelial cells, fibroblastsAbstract
Non-healing or chronic wounds are widely distributed complications of several pathologic states. A study of healing mechanisms requires an adequate animal model of such wounds. Use of rodents, one of the most available laboratory animals, is linked with some problems: wound edge contraction that precedes re-epithelialization. Mitomycin C (MMC), as a pharmacological inhibitor of cell proliferation, can be used in chronic wound modelling.
The aim. The objective of the research was to create a model of non-healing (chronic) wound by surgically limiting its contraction and inhibiting recovery rate with the pharmaceutical agent mitomycin C (MMC).
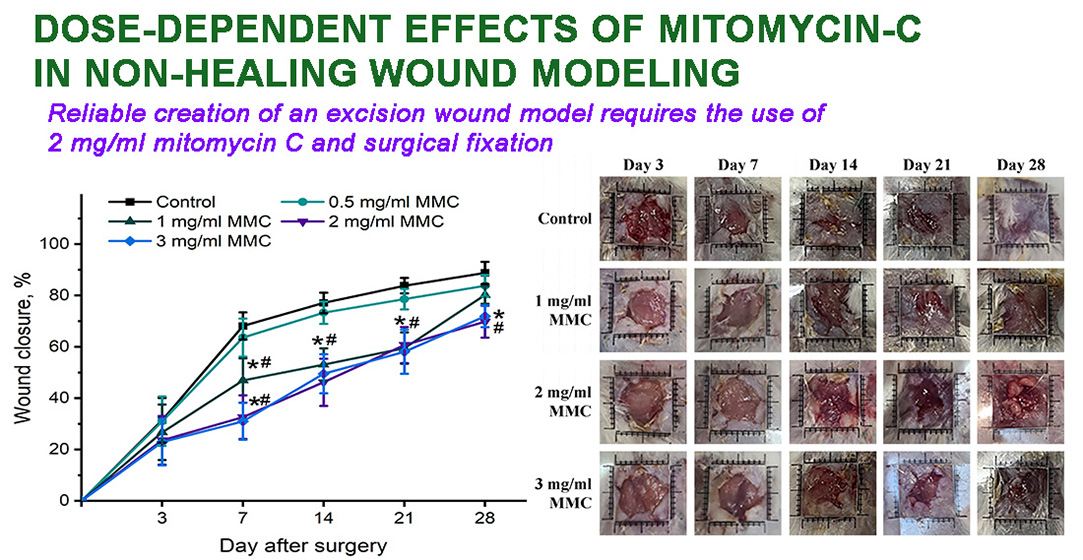
Materials and methods. Male Balb/c mice were used. Two layers of skin were pierced through, resulting in the simultaneous formation of two wounds (~0.6 cm3), whose edges were sutured surgically to hinder their contraction. Wounds were additionally treated with 0.5, 1, 2, and 3 mg/ml MMC. The delay of healing was assessed by measuring wound area and by morphological and histological examination.
Results. The application of 2 and 3 mg/ml MMC for surgically fortified excision wounds resulted in a significantly increased area by day 21 and 28 compared with groups treated with lower doses. Also, wounds had loci of necrosis and infiltration. Delayed re-epithelialization and irregular collagen fibres were observed histologically after treatment with 2 and 3 mg/ml. Considering the absence of differences between wounds treated with 2 and 3 mg/ml MMC and its potential toxic effects, 2 mg/ml was recommended for non-healing wound modelling.
Conclusions. An optimal model of non-healing (chronic) wound was created. The main aspects of the murine model can be outlined as follows: the use of surgical fixation of wound edges to a dense polymer base and treatment with 2 mg/ml MMC
Supporting Agency
- National research foundation of Ukraine (Grant No. 2021.01/0276)
References
- Guidance for industry: Chronic cutaneous ulcer and burn wounds – developing products for treatment (2001). Wound Repair and Regeneration, 9 (4), 258–268. https://doi.org/10.1046/j.1524-475x.2001.00258.x
- Bowers, S., Franco, E. (2020). Chronic Wounds: Evaluation and Management. American family physician, 101 (3), 159–166. Available at: https://www.aafp.org/pubs/afp/issues/2020/0201/p159.pdf
- Sen, C. K. (2021). Human Wound and Its Burden: Updated 2020 Compendium of Estimates. Advances in Wound Care, 10 (5), 281–292. https://doi.org/10.1089/wound.2021.0026
- Martinengo, L., Olsson, M., Bajpai, R., Soljak, M., Upton, Z., Schmidtchen, A. et al. (2019). Prevalence of chronic wounds in the general population: systematic review and meta-analysis of observational studies. Annals of Epidemiology, 29, 8–15. https://doi.org/10.1016/j.annepidem.2018.10.005
- Wall, I. B., Moseley, R., Baird, D. M., Kipling, D., Giles, P., Laffafian, I. et al. (2008). Fibroblast Dysfunction Is a Key Factor in the Non-Healing of Chronic Venous Leg Ulcers. Journal of Investigative Dermatology, 128 (10), 2526–2540. https://doi.org/10.1038/jid.2008.114
- Zhao, R., Liang, H., Clarke, E., Jackson, C., Xue, M. (2016). Inflammation in Chronic Wounds. International Journal of Molecular Sciences, 17 (12), 2085. https://doi.org/10.3390/ijms17122085
- Eming, S. A., Martin, P., Tomic-Canic, M. (2014). Wound repair and regeneration: Mechanisms, signaling, and translation. Science Translational Medicine, 6(265). https://doi.org/10.1126/scitranslmed.3009337
- Rogulska, O., Tykhvynska, O., Revenko, O., Grischuk, V., Mazur, S., Volkova, N. et al. (2019). Novel Cryopreservation Approach Providing Off-the-Shelf Availability of Human Multipotent Mesenchymal Stromal Cells for Clinical Applications. Stem Cells International, 2019, 1–11. https://doi.org/10.1155/2019/4150690
- Tykhvynska, O. A., Rogulska, O. Yu., Petrenko, Y. A. (2017). Mesenchymal Stromal Cells Within Fibrin Gel Stimulate Healing of Full-Thickness Wounds in Mice. Problems of Cryobiology and Cryomedicine, 27 (2), 171–171. https://doi.org/10.15407/cryo27.02.171
- Papageorgopoulou, C., Nikolakopoulos, K., Seretis, C. (2022). Hyperthermic Intraperitoneal Chemotherapy with Mitomycin C versus Oxaliplatin after Cytoreductive Surgery for the Treatment of Peritoneal Metastases of Colorectal Cancer Origin. Chirurgia, 117 (3), 266–277. https://doi.org/10.21614/chirurgia.2708
- Paz, M. M., Zhang, X., Lu, J., Holmgren, A. (2012). A New Mechanism of Action for the Anticancer Drug Mitomycin C: Mechanism-Based Inhibition of Thioredoxin Reductase. Chemical Research in Toxicology, 25 (7), 1502–1511. https://doi.org/10.1021/tx3002065
- Snodgrass, R. G., Collier, A. C., Coon, A. E., Pritsos, C. A. (2010). Mitomycin C Inhibits Ribosomal RNA. Journal of Biological Chemistry, 285 (25), 19068–19075. https://doi.org/10.1074/jbc.m109.040477
- Ribeiro, F. de A. Q., Guaraldo, L., de Pádua Borges, J., Vianna, M. R., Eckley, C. A. (2008). Study of Wound Healing in Rats Treated with Topical and Injected Mitomycin C. Annals of Otology, Rhinology & Laryngology, 117 (10), 786–790. https://doi.org/10.1177/000348940811701015
- Sewall, G. K., Robertson, K. M., Connor, N. P., Heisey, D. M., Hartig, G. K. (2003). Effect of Topical Mitomycin on Skin Wound Contraction. Archives of Facial Plastic Surgery, 5 (1), 59–62. https://doi.org/10.1001/archfaci.5.1.59
- Eyibilen, A., Güven, M., Aladağ, İ., Kesici, H., Koç, S., Gürbüzler, L. et al. (2011). Does mitomycin-C increase collagen turnover as a modulator of wound healing in tracheostomyzed rats? The Turkish Journal of Ear Nose and Throat, 21 (3), 154–158. https://doi.org/10.5606/kbbihtisas.2011.017
- de Andrade Quintanilha Ribeiro, F., de Pádua Borges, J., Guaraldo, L., Vianna, M. R. (2008). Study of wound healing in rats treated with topical and injected mitomycin-C. Brazilian Journal of Otorhinolaryngology, 74 (3), 328–330. https://doi.org/10.1016/s1808-8694(15)30563-2
- Naldaiz‐Gastesi, N., Bahri, O. A., López de Munain, A., McCullagh, K. J. A., Izeta, A. (2018). The panniculus carnosus muscle: an evolutionary enigma at the intersection of distinct research fields. Journal of Anatomy, 233 (3), 275–288. https://doi.org/10.1111/joa.12840
- Krzyszczyk, P., Schloss, R., Palmer, A., Berthiaume, F. (2018). The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-wound Healing Phenotypes. Frontiers in Physiology, 9. https://doi.org/10.3389/fphys.2018.00419
- Ariawan, A., Wicaksana, A., Rifqi Fauzi, A., Seswandhana, R. (2018). Chronic wound mitomycin-c-induced animal models. Journal of Thee Medical Sciences (Berkala Ilmu Kedokteran), 50 (2). https://doi.org/10.19106/jmedsci005002201803
- Nagano, H., Suematsu, Y., Takuma, M., Aoki, S., Satoh, A., Takayama, E. et al. (2021). Enhanced cellular engraftment of adipose-derived mesenchymal stem cell spheroids by using nanosheets as scaffolds. Scientific Reports, 11 (1). https://doi.org/10.1038/s41598-021-93642-6
- Su, C., Sui, T., Zhang, X., Zhang, H., Cao, X. (2012). Effect of topical application of mitomycin-C on wound healing in a postlaminectomy rat model: An experimental study. European Journal of Pharmacology, 674 (1), 7–12. https://doi.org/10.1016/j.ejphar.2011.10.028
- Lampus, H. F., Kusmayadi, D. D., Nawas, B. A. (2015). The influence of topical mitomycin-C on total fibroblasts, epithelialization, and collagenization in anoplasty wound healing in Wistar rats. Journal of Pediatric Surgery, 50 (8), 1347–1351. https://doi.org/10.1016/j.jpedsurg.2015.03.059
- Saputro, I. B. W., Primitasari, Y., Fatmariyanti, S. (2022). The Effect of The Mitomycin C on Anophthalmic Contracted Socket (Literature Review). International Journal of Scientific Advances, 3 (3). https://doi.org/10.51542/ijscia.v3i3.5
- Xie, H., Wang, B., Shen, X., Qin, J., Jiang, L., Yu, C. et al. (2017). MMC controlled-release membranes attenuate epidural scar formation in rat models after laminectomy. Molecular Medicine Reports, 15 (6), 4162–4168. https://doi.org/10.3892/mmr.2017.6531
- Yoshimoto, M., Saito, M., Tada, T., Takahashi, K., Kasumi, F. (2001). Unexpected increase in the bone marrow toxicity of mitomycin C (MMC). British Journal of Cancer, 84 (5), 736–736. https://doi.org/10.1054/bjoc.2000.1644
- Serretta, V., Scalici Gesolfo, C., Alonge, V., Di Maida, F., Caruana, G. (2016). Mitomycin C from Birth to Adulthood. Urologia Journal, 83, 2–6. https://doi.org/10.5301/uro.5000195
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Oleksandr Pakhomov, Olena Revenko, Daria Cherkashina, Galyna Bozhok, Natalia Trufanova, Svitlana Mazur, Oleksandr Petrenko

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.







